Publications
Activin A
Van Nerum, K., Wenzel, A., Argemi-Muntadas, L. et al.
α-Ketoglutarate promotes trophectoderm induction and maturation from naive human embryonic stem cells
Garitta E
Modelling Cholestasis in vitro Using Hepatocytes Derived from Human Induced Pluripotent Stem Cells
Huang, T et al.
Inhibition of PRC2 enables self-renewal of blastoid-competent naive pluripotent stem cells from chimpanzee
Zorzan I, Pellegrini M, Arboit M et al.
The transcriptional regulator ZNF398 mediates pluripotency and epithelial character downstream of TGF-beta in human PSCs
From the lab of Graziano Martello, University of Padua
Beucher A, Miguel-Escalada I, Balboa D et al.
The HASTER lncRNA promoter is a cis-acting transcriptional stabilizer of HNF1A
From the lab of Jorge Ferrer, Centre for Genomic Regulation (CRG)
Bao M, Cornwall-Scoones J, Sanchez-Vasquez E et al.
Stem cell-derived synthetic embryos self-assemble by exploiting cadherin codes and cortical tension
From the lab of Magdalena Zernicka-Goetz, University of Cambridge
Meek S, Watson T, Eory L et al.
Stem cell-derived porcine macrophages as a new platform for studying host-pathogen interactions
From the lab of Tom Burdon, University of Edinburgh
Azami T, Theeuwes B, Ton M-LN et al.
STAT3 signalling enhances tissue expansion during postimplantation mouse development
Balmas E, Ratto ML, Snijders KE et al.
Single Cell Transcriptional Perturbome in Pluripotent Stem Cell Models
Guo M, Wu J, Chen C et al.
Self-renewing human naïve pluripotent stem cells dedifferentiate in 3D culture and form blastoids spontaneously
From the lab of José Silva, Guangzhou Laboratory
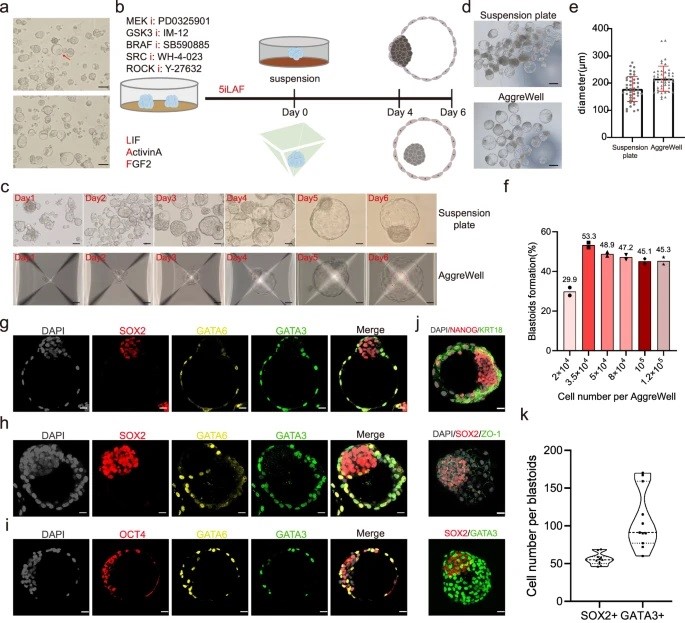
A huge challenge in understanding human early embryo cell fate is due to limited access and ethical concerns. Recent research, however, from José C. R. Silva’s lab at Guangzhou National Laboratory, drawing from single-cell sequencing, suggests a conserved lineage specification process between human and mouse embryos. Blastoids, emerging models for early embryo development, generated solely from hnPSCs, offer insights into blastocyst formation without altering culture conditions. Self-renewing human naïve pluripotent stem cells (hnPSCs) spontaneously form blastoids in 3D culture, mimicking early human blastocysts. This process, mediated by the GSK3 inhibitor IM-12 in 5iLAF medium, involves upregulation of oxidative phosphorylation genes. hnPSCs dedifferentiate into E5 embryo-like intermediates, expressing SOX2/OCT4 and GATA6, which specify trophoblast fate by day 3, coinciding with blastoid formation. This was a fantastic paper to read as it is clear how this spontaneous blastoid formation highlights the importance of culture conditions and provides a new platform to study human embryo development in vitro, potentially reshaping our understanding of hnPSCs and embryo development.
Farbergshagen, AC
Role of mechanotransduction in pancreatic endocrine cell fate acquisition in SC-islets
DOI: Thesis
Blackford SJI, Yu TTL, Norman MDA et al.
Validation of Current Good Manufacturing Practice Compliant Human Pluripotent Stem Cell-Derived Hepatocytes for Cell-Based Therapy
From the lab of Tamir Rashid, Kings College London
Truszkowski L, Bottini S, Bianchi S et al.
Refined home-brew media for cost-effective, weekend-free hiPSC culture and genetic engineering
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
Used:
- Recombinant human activin A protein (Qk001)
- Recombinant human FGF-2 (145 aa) protein (Qk025)
- Recombinant human FGF-2 (154 aa) protein (Qk027)
- Recombinant human BMP-4 protein (Qk038)
- Recombinant human NRG-1 protein (Qk045)
- Recombinant FGF2-G3 (145 aa) protein (Qk052)
- Recombinant FGF2-G3 (154 aa) protein (Qk053)
- Recombinant human TGF-β3 protein (Qk054)
- Recombinant human TGF-β1 PLUS™ protein (Qk010)
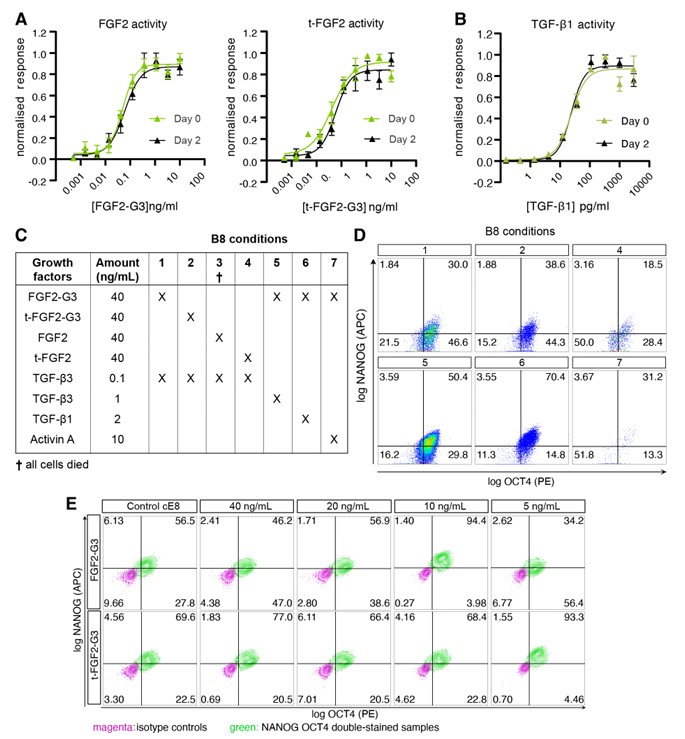
Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
Militi S, Nibhani R, Jalali M and Pauklin S.
RBL2-E2F-GCN5 guide cell fate decisions during tissue specification by regulating cell-cycle-dependent fluctuations of non-cell-autonomous signaling
From the lab of Siim Pauklin, University of Oxford
Rosa VS, Sato N and Shahbazi MN et al.
Protocol for generating a 3D culture of epiblast stem cells
BDNF
Magarotto M, Gawne RT, Vilkaite G, Mason AS, Chen H-J
Familial ALS/FTD-associated RNA-Binding deficient TDP-43 mutants cause neuronal and synaptic dysregulation in vitro
Nuhu-Soso L, Denton H, Goffin DL, Hahn I and Evans GJO.
Neuronal differentiation enhances a cytoplasmic pool of tousled-like kinase 2 (TLK2)
Buchner F, Dokuzluoglu Z, Thomas J et al.
Synchronous 3D patterning of diverse CNS progenitors generates motor neurons of broad axial identity
From the lab of Dr. Natalia Rodriguez-Muela, German Center for Neurodegenerative Diseases e.V. (DZNE)
Used:
Chen HJC, Yang A, Mazzaferro S et al.
Profiling human hypothalamic neurons reveals a candidate combination drug therapy for weight loss
From the labs of John C. Marioni, European Bioinformatics Institute and Florian T. Merkle, University of Cambridge
Page T, Musi CA, Bakker SE et al.
Parkinson’s associated protein DJ-1 regulates intercellular communication via extracellular vesicles in oxidative stress
Pivoňková H, Sitnikov S, Kamen Y et al.
Heterogeneity in oligodendrocyte precursor cell proliferation is dynamic and driven by passive bioelectrical properties
From the lab of Ragnhildur Thóra Káradóttir, Cambridge Stem Cell Institute
Macarelli V, Harding EC, Gershlick DC, Merkle FT.
A short sequence targets transmembrane proteins to primary cilia
From the lab of Florian Merkle, University of Cambridge
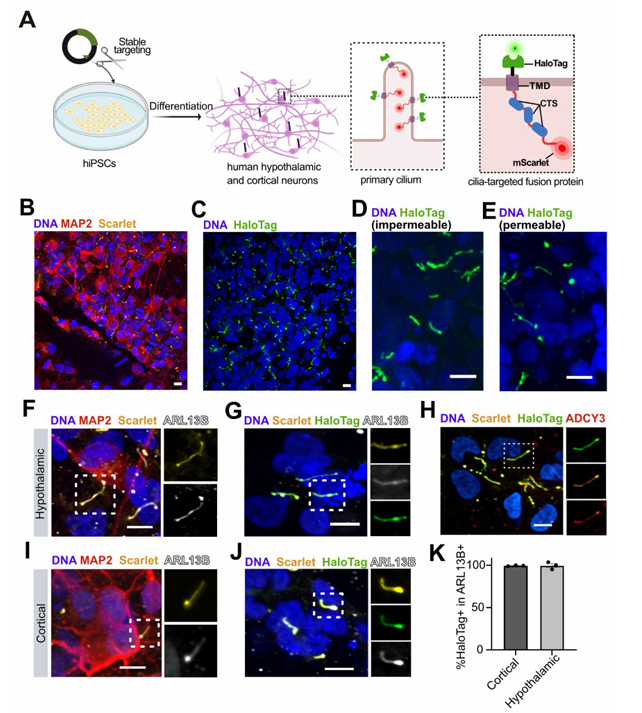
Macarelli et al demonstrate the importance of using the right cells and tools for the study of neural stem cell models in this recent publication. They explored the use of different ciliary targeting sequences to fluorescently label and measure cilia. This allows the study of the structure and function of these important sensory and signaling structures in differentiated neural cells. An exciting addition to the knowledge of tools to study neural differentiation from the lab of Dr Florian Merkle at the Cambridge Stem Cell Institute.
Agarwal D et al.
Human retinal ganglion cell neurons generated by synchronous BMP inhibition and transcription factor mediated reprogramming.
From the lab of Karl Wahlin, University of California San Diego
Agarwal D & Wahlin K
Differentiation of RGC Induced Neurons (RGC-iNs).
From the lab of Karl Wahlin, University of California San Diego
BMP-2
Huang TC, Wang YF, Vazquez-Ferrer E et al.
Sex-specific chromatin remodelling safeguards transcription in germ cells
From the lab of Petra Hajkova, MRC London Institute of Medical Sciences
Tan J, Virtue S, Norris DM et al.
Limited oxygen in standard cell culture alters metabolism and function of differentiated cells
From the lab of Daniel J. Fazakerley, University of Cambridge

We all know our stem cell cultures are sensitive and high maintenance, we feed them, keep them warm to make sure we get the most accurate, reproducible data. The composition of their media is important, we need the right growth factors, but this week’s Friday read highlights that their exposure to oxygen can be an important factor in the differentiation and function of cellular models. In this very interesting read from the MRC Institute of Metabolic Science they have investigated the impact of oxygen diffusion and media volume on hypoxia-related transcriptional changes in stem cell cultures. They found that decreasing the media volume decreased lactate production and HIF1α expression and increased the functionality of adipocyte, hiPSC-derived hepatocytes and hiPSC-derived cardiac organoid cultures.
Luo L, Foster NC, Man KL et al.
Hydrostatic pressure promotes chondrogenic differentiation and microvesicle release from human embryonic and bone marrow stem cells
Stucchi S, Sepulveda-Rincon LP, Dion C et al.
High resolution multi-scale profiling of embryonic germ cell-like cells derivation reveals pluripotent state transitions in humans
Yimiti D, Uchibe K, Toriyama M et al.
CD1530, selective RARγ agonist, facilitates Achilles tendon healing by modulating the healing environment including less chondrification in a mouse model
BMP-4
Barbieri E and Chambers I
OTX2 controls chromatin accessibility to direct somatic versus germline differentiation
From the lab of Professor Ian Chambers, The University of Edinburgh
Azami T, Theeuwes B, Ton M-LN et al.
STAT3 signalling enhances tissue expansion during postimplantation mouse development
Balmas E, Ratto ML, Snijders KE et al.
Single Cell Transcriptional Perturbome in Pluripotent Stem Cell Models
Truszkowski L, Bottini S, Bianchi S et al.
Refined home-brew media for cost-effective, weekend-free hiPSC culture and genetic engineering
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
Used:
- Recombinant human activin A protein (Qk001)
- Recombinant human FGF-2 (145 aa) protein (Qk025)
- Recombinant human FGF-2 (154 aa) protein (Qk027)
- Recombinant human BMP-4 protein (Qk038)
- Recombinant human NRG-1 protein (Qk045)
- Recombinant FGF2-G3 (145 aa) protein (Qk052)
- Recombinant FGF2-G3 (154 aa) protein (Qk053)
- Recombinant human TGF-β3 protein (Qk054)
- Recombinant human TGF-β1 PLUS™ protein (Qk010)

Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
Drozd AM, Mariani L, Guo X, Goitea V, Menezes NA and Ferretti E.
Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac progenitor Specification during Mouse Gastrulation
Stucchi S, Sepulveda-Rincon LP, Dion C et al.
High resolution multi-scale profiling of embryonic germ cell-like cells derivation reveals pluripotent state transitions in humans
EGF
Van Nerum, K., Wenzel, A., Argemi-Muntadas, L. et al.
α-Ketoglutarate promotes trophectoderm induction and maturation from naive human embryonic stem cells
Christensen JB, Donovan APA, Marzieh Moradi M et al.
A conserved differentiation program facilitates inhibitory neuron production in the developing mouse and human cerebellum
Żylicz J, van Nerum K, Wenzel A et al.
Metabolic rewiring underpins human trophoblast induction
From the lab of Jan Żylicz, University of Copenhagen, Denmark
Darrigrand J-F, Isaacson A and Spagnoli FM
Generation of human iPSC-derived pancreatic organoids to study pancreas development and disease
Rossignoli G, Oberhuemer M, Brun IS et al.
Feeder-free culture of naive human pluripotent stem cells retaining embryonic, extraembryonic and blastoid generation potential
FGF-10
Iyer DP, Khoei HH, van der Weijden VA et al.
mTOR activity paces human blastocyst stage developmental progression
Agarwal R, Dittmar T, Beer HD et al.
Human epidermis organotypic cultures, a reproducible system recapitulating the epidermis in vitro
Darrigrand J-F, Isaacson A and Spagnoli FM
Generation of human iPSC-derived pancreatic organoids to study pancreas development and disease
FGF-2
Christensen JB, Donovan APA, Marzieh Moradi M et al.
A conserved differentiation program facilitates inhibitory neuron production in the developing mouse and human cerebellum
Wang Y, Siebzehnrubl D, Weller M et al.
Vortioxetine: A Potential Drug for Repurposing for Glioblastoma Treatment via a Microsphere Local Delivery System
From the lab of Ben Newland, Cardiff University, UK
Huang, T et al.
Inhibition of PRC2 enables self-renewal of blastoid-competent naive pluripotent stem cells from chimpanzee
Zorzan I, Pellegrini M, Arboit M et al.
The transcriptional regulator ZNF398 mediates pluripotency and epithelial character downstream of TGF-beta in human PSCs
From the lab of Graziano Martello, University of Padua
Buchner F, Dokuzluoglu Z, Thomas J et al.
Synchronous 3D patterning of diverse CNS progenitors generates motor neurons of broad axial identity
From the lab of Dr. Natalia Rodriguez-Muela, German Center for Neurodegenerative Diseases e.V. (DZNE)
Used:
Meek S, Watson T, Eory L et al.
Stem cell-derived porcine macrophages as a new platform for studying host-pathogen interactions
From the lab of Tom Burdon, University of Edinburgh
Balmas E, Ratto ML, Snijders KE et al.
Single Cell Transcriptional Perturbome in Pluripotent Stem Cell Models
Truszkowski L, Bottini S, Bianchi S et al.
Refined home-brew media for cost-effective, weekend-free hiPSC culture and genetic engineering
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
Used:
- Recombinant human activin A protein (Qk001)
- Recombinant human FGF-2 (145 aa) protein (Qk025)
- Recombinant human FGF-2 (154 aa) protein (Qk027)
- Recombinant human BMP-4 protein (Qk038)
- Recombinant human NRG-1 protein (Qk045)
- Recombinant FGF2-G3 (145 aa) protein (Qk052)
- Recombinant FGF2-G3 (154 aa) protein (Qk053)
- Recombinant human TGF-β3 protein (Qk054)
- Recombinant human TGF-β1 PLUS™ protein (Qk010)

Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
Rosa VS, Sato N and Shahbazi MN et al.
Protocol for generating a 3D culture of epiblast stem cells
Drozd AM, Mariani L, Guo X, Goitea V, Menezes NA and Ferretti E.
Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac progenitor Specification during Mouse Gastrulation
Page T, Musi CA, Bakker SE et al.
Parkinson’s associated protein DJ-1 regulates intercellular communication via extracellular vesicles in oxidative stress
Tan J, Virtue S, Norris DM et al.
Oxygen is a critical regulator of cellular metabolism and function in cell culture
Iyer DP, Khoei HH, van der Weijden VA et al.
mTOR activity paces human blastocyst stage developmental progression
Beltran-Rendon C, Price CJ, Glen K et al.
Modeling the selective growth advantage of genetically variant human pluripotent stem cells to identify opportunities for manufacturing process control
From the lab of Robert Thomas, Loughborough University
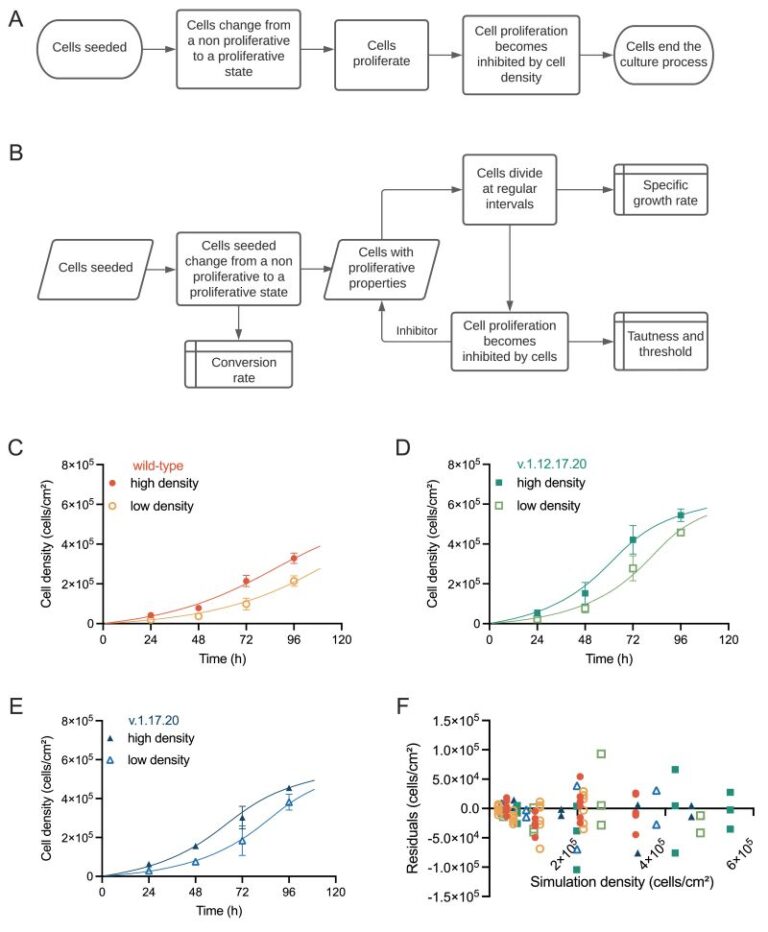
Robert Thomas’s lab at Loughborough University has analyzed growth dynamics between commonly occurring genetically variant hPSCs and their counterpart wild-type cells in culture cells using proprietary computational modelling, allowing the identification of critical process parameters that drive critical quality attributes when genetically variant cells are present within the system This fascinating paper highlights how the system parameters controlling independent growth behavior of wild-type and genetic variant populations are altered when both populations exist within a co-culture environment by introducing an ordinary differential equation (ODE) framework. Findings reveal that variant cells exhibit selective growth and competitive advantage, influencing the behavior of wild-type cells, particularly at higher culture densities. This computational model offers opportunities for defining operational protocols and timely detection of emerging variants, crucial for product release and risk management. It is clear to see the importance as it demonstrates the utility of computational models in understanding complex biological systems and informing manufacturing practices in hPSC-based therapies.
GDF-15
Ley-Ngardigal S, Claverol S, Sobilo L et al.
Repression of oxidative phosphorylation by NR2F2, MTERF3 and GDF15 in human skin under high-glucose stress
From the lab of Rodrigue Rossignol, University of Bordeaux
Karusheva Y, Ratcliff M, Mörseburg A et al.
The Common H202D Variant in GDF-15 Does Not Affect Its Bioactivity but Can Significantly Interfere with Measurement of Its Circulating Levels
From the lab of Professor Sir Stephen O’Rahilly, University of Cambridge
Fejzo M, Rocha N, Cimino I et al.
GDF15 linked to maternal risk of nausea and vomiting during pregnancy
From the lab of Stephen O’Rahilly, University of Cambridge
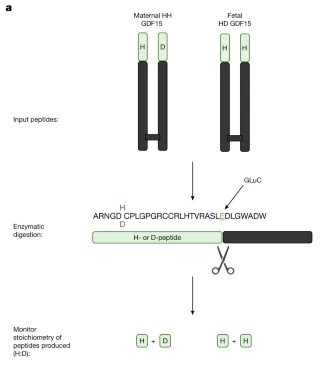
The recent publication from Fejzo et al. discusses the role of GDF-15, an intriguing protein acting on the brainstem, in nausea and vomiting during pregnancy, particularly in hyperemesis gravidarum (HG). This study finds that both fetal production of GDF-15 and maternal sensitivity to GDF-15 significantly contribute to the risk of HG with higher levels of GDF15 in maternal blood associated with vomiting during pregnancy and HG. Genetic variants affecting GDF-15 levels influence the risk of HG, with low levels increasing the risk and high levels decreasing it. This was an excellent read which suggests a putative causal role for fetally derived GDF-15 in pregnancy-related nausea and vomiting, with maternal sensitivity influenced by pre-pregnancy exposure and shows great promise for potential avenues of treatment and prevention of HG by blocking GDF-15 action in the pregnant mother.
Fejzo M et al.
Fetally-encoded GDF15 and maternal GDF15 sensitivity are major determinants of nausea and vomiting in human pregnancy
From the lab of Stephen O’Rahilly, University of Cambridge, UK and Nicholas Mancuso, University of Southern California, USA.
Jeromson S, Akcan M, Baranowski B, Arbeau M, Bellucci A, Wright DC.
Daily GDF15 treatment has sex‐specific effects on body weight and food intake and does not enhance the effects of voluntary physical activity in mice
From the lab of David Wright, University of British Columbia
Cimino I, Kim H, Tung YCL et al.
Activation of the hypothalamic–pituitary–adrenal axis by exogenous and endogenous GDF15
From the lab of Stephen O’Rahilly, University of Cambridge
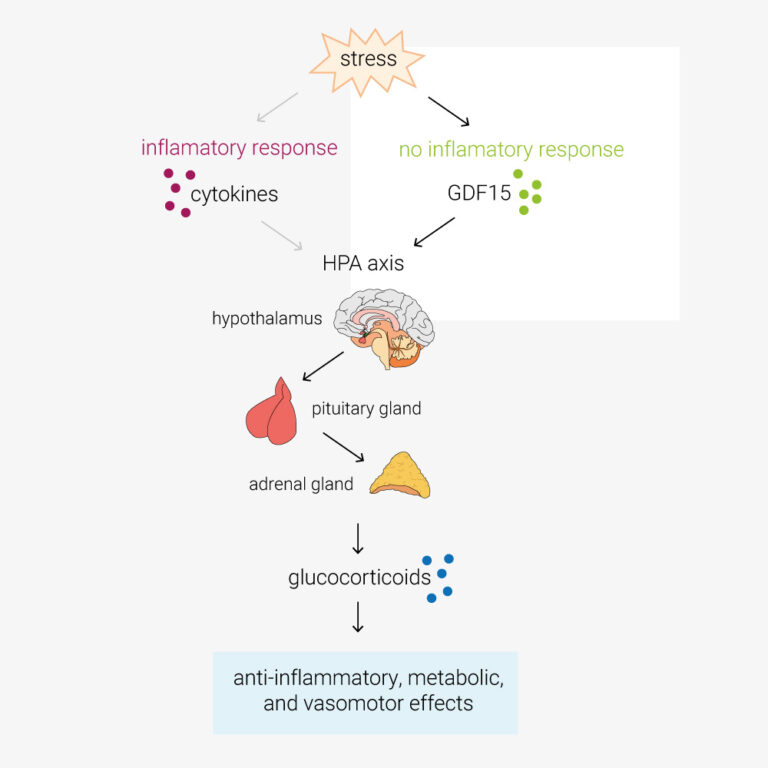
In this paper, Cinimo et al. explore the role of the TGFβ-family protein GDF15 in activation of the hypothalamic–pituitary–adrenal (HPA) axis. During infection, cytokines such as TNFα/β, IL-1 and IL-6, activate the HPA axis. This increases circulating glucocorticoids, which have anti-inflammatory, metabolic, and vasomotor effects. However, O’Rahilly lab have determined that in response to stimuli such as toxins, which don’t provoke an inflammatory response, the primary activator of the HPA axis is GDF15. GDF15 is an intriguing protein also being explored as an anti-obesity therapeutic target, these findings may have a pivotal impact on future clinical study design and open new avenues of investigation. Certainly cool science!
GDNF
Buchner F, Dokuzluoglu Z, Thomas J et al.
Synchronous 3D patterning of diverse CNS progenitors generates motor neurons of broad axial identity
From the lab of Dr. Natalia Rodriguez-Muela, German Center for Neurodegenerative Diseases e.V. (DZNE)
Used:
Page T, Musi CA, Bakker SE et al.
Parkinson’s associated protein DJ-1 regulates intercellular communication via extracellular vesicles in oxidative stress
Agarwal D et al.
Human retinal ganglion cell neurons generated by synchronous BMP inhibition and transcription factor mediated reprogramming.
From the lab of Karl Wahlin, University of California San Diego
Agarwal D & Wahlin K
Differentiation of RGC Induced Neurons (RGC-iNs).
From the lab of Karl Wahlin, University of California San Diego
Gremlin
Sato N, Rosa VS, Makhlouf A et al.
Basal delamination during mouse gastrulation primes pluripotent cells for differentiation
From the lab of Marta Shahbazi, MRC Laboratory of Molecular Biology, Cambridge
HGF
Iyer DP, Khoei HH, van der Weijden VA et al.
mTOR activity paces human blastocyst stage developmental progression
KGF (FGF-7)
Darrigrand J-F, Isaacson A and Spagnoli FM
Generation of human iPSC-derived pancreatic organoids to study pancreas development and disease
LIF
Van Nerum, K., Wenzel, A., Argemi-Muntadas, L. et al.
α-Ketoglutarate promotes trophectoderm induction and maturation from naive human embryonic stem cells
Balayo T, Lunn S, Pascual-Mas P et al.
N2B27 media formulations influence gastruloid development
Huang, T et al.
Inhibition of PRC2 enables self-renewal of blastoid-competent naive pluripotent stem cells from chimpanzee
Dinarello A, Betto RM, Diamante L et al.
STAT3 and HIF1α cooperatively mediate the transcriptional and physiological responses to hypoxia
From the lab of Graziano Martello and Francesco Argenton, University of Padova
Guo M, Wu J, Chen C et al.
Self-renewing human naïve pluripotent stem cells dedifferentiate in 3D culture and form blastoids spontaneously
From the lab of José Silva, Guangzhou Laboratory

A huge challenge in understanding human early embryo cell fate is due to limited access and ethical concerns. Recent research, however, from José C. R. Silva’s lab at Guangzhou National Laboratory, drawing from single-cell sequencing, suggests a conserved lineage specification process between human and mouse embryos. Blastoids, emerging models for early embryo development, generated solely from hnPSCs, offer insights into blastocyst formation without altering culture conditions. Self-renewing human naïve pluripotent stem cells (hnPSCs) spontaneously form blastoids in 3D culture, mimicking early human blastocysts. This process, mediated by the GSK3 inhibitor IM-12 in 5iLAF medium, involves upregulation of oxidative phosphorylation genes. hnPSCs dedifferentiate into E5 embryo-like intermediates, expressing SOX2/OCT4 and GATA6, which specify trophoblast fate by day 3, coinciding with blastoid formation. This was a fantastic paper to read as it is clear how this spontaneous blastoid formation highlights the importance of culture conditions and provides a new platform to study human embryo development in vitro, potentially reshaping our understanding of hnPSCs and embryo development.
Li H, Chang L, Huang J and Silva JCR.
Protocol for generating mouse morula-like cells resembling 8- to 16-cell stage embryo cells
From the lab of José Silva, Guangzhou Laboratory
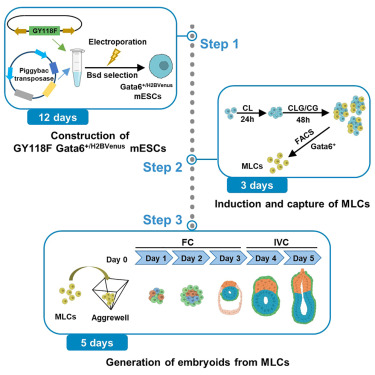
Generating cell types with properties of embryo cells with full developmental potential is of great biological importance. This paper describe steps for induction and isolation of MLCs by sorting. They explained the procedures for segregating MLCs into blastocyst cell fates and how to create embryo-like structures from them. This system provides a valuable stem-cell-based embryo model to study early embryo development.
Rosa VS, Sato N and Shahbazi MN et al.
Protocol for generating a 3D culture of epiblast stem cells
Drozd AM, Mariani L, Guo X, Goitea V, Menezes NA and Ferretti E.
Progesterone Receptor Modulates Extraembryonic Mesoderm and Cardiac progenitor Specification during Mouse Gastrulation
Krammer T and Tanaka EM
Neural tube organoid generation: a robust and reproducible protocol from single mouse embryonic stem cells
From the lab of Elly M. Tanaka, Francis Crick Institute and the Research Institute of Molecular Pathology (IMP) of Vienna
Hennessy MJ, Fulton T, Turner DA and Steventon B
Negative feedback on Retinoic Acid by Brachyury guides gastruloid symmetry-breaking
From the labs of David A. Turner, University of Liverpool and Ben Steventon, University of Cambridge
Borkowska M, Leitch HG.
Mouse Primordial Germ Cells: In Vitro Culture and Conversion to Pluripotent Stem Cell Lines
Krammer T, Stuart HT, Gromberg E et al.
Mouse neural tube organoids self-organize floorplate through BMP-mediated cluster competition
From the labs of James Briscoe and Elly M. Tanaka, Francis Crick Institute and the Research Institute of Molecular Pathology (IMP) of Vienna

This paper gives us insight into the complex regulatory interactions between BMP-4 and noggin during neural tube development. Neural tube organoids from embryonic stem cells will organize into floorplates, without the usual inducers present in the developing embryo. They used mouse neural tube organoids and by identifying the floorplate marker FOXA2 they showed the importance of regulation of BMP-4 signaling through noggin. Noggin mutation reduced neural tube organization and floorplate formation in vitro and in vivo.
Żylicz J, van Nerum K, Wenzel A et al.
Metabolic rewiring underpins human trophoblast induction
From the lab of Jan Żylicz, University of Copenhagen, Denmark
Arboit M, Zorzan I, Pellegrini M et al.
KLF7 is a general inducer of human pluripotency
From the lab of Elena Carbognin and Graziano Martello, University of Padua
Klobučar T, Novljan J, Iosub IA at el.
Integrative analysis of higher-order transcriptome organisation and RNA condensation principles
From the lab of Miha Modic, National Institute of Chemistry, Ljubljana, Slovenia
Noggin
Lumibao JC, Okhovat SR, Peck KL et al.
The effect of extracellular matrix on the precision medicine utility of pancreatic cancer patient-derived organoids
From the lab of Dannielle Engle, Salk Institute for Biological Studies

A promising application of organoids, in this case patient-derived organoids (PDOs), is to profile the therapeutic sensitivity of tumors to inform clinical decisions and to stratify patients in clinical trials more effectively. Recently there have been positive results from clinical trials giving a glimmer of the potential impact of these technologies. However, a concern is how reproducible these technologies are, particularly as most still rely on animal-derived basement membrane extracts, where batch variation is a sector-wide concern. This recent publication shows consistent drug responses are observed when different sources of commercial BME were compared. This is reassuring and speaks to how robust these approaches can be.
Bergmann S, Penfold CA, Slatery E et al.
Spatial profiling of early primate gastrulation in utero
From the lab of Thorsten E. Boroviak, University of Cambridge
Osorio-Vasquez V, Lumibao JC, Peck KL et al.
Identification of molecular and functional subtypes using chronic pancreatitis patient-derived organoid models
From the lab of Dannielle D. Engle, Salk Institute for Biological Studies
Agarwal R, Dittmar T, Beer HD et al.
Human epidermis organotypic cultures, a reproducible system recapitulating the epidermis in vitro
Feofanov M, Daubner GM, Saltalamacchia A et al.
Discovery and optimization of a guanylhydrazone-based small molecule to replace bFGF for cell culture applications
NRG-1
Truszkowski L, Bottini S, Bianchi S et al.
Refined home-brew media for cost-effective, weekend-free hiPSC culture and genetic engineering
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
Used:
- Recombinant human activin A protein (Qk001)
- Recombinant human FGF-2 (145 aa) protein (Qk025)
- Recombinant human FGF-2 (154 aa) protein (Qk027)
- Recombinant human BMP-4 protein (Qk038)
- Recombinant human NRG-1 protein (Qk045)
- Recombinant FGF2-G3 (145 aa) protein (Qk052)
- Recombinant FGF2-G3 (154 aa) protein (Qk053)
- Recombinant human TGF-β3 protein (Qk054)
- Recombinant human TGF-β1 PLUS™ protein (Qk010)

Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
NT-3
Liu Y, Li X, Xu H et al.
Spinal cord stimulation induces Neurotrophin-3 to improve diabetic foot disease
PDGF
Mulholland KE, Bourguet M, Cheng N et al.
Pervanadate-induced oxidation relieves autoinhibition of SRC protein tyrosine kinase
Qkine
Barber L, Spicer C.
The effect of pyridinecarboxaldehyde functionalisation on reactivity and N-terminal protein modification
From the lab of Christopher Spicer, University of York
Karusheva Y, Ratcliff M, Mörseburg A et al.
The Common H202D Variant in GDF-15 Does Not Affect Its Bioactivity but Can Significantly Interfere with Measurement of Its Circulating Levels
From the lab of Professor Sir Stephen O’Rahilly, University of Cambridge
Barber LJ, Yates NDJ, Fascione MA et al.
Selectivity and stability of N-terminal targeting protein modification chemistries
Venkatesan M, Semper C, Skrivergaard S et al.
Recombinant production of growth factors for application in cell culture
From the lab of Alexi Savchenko, University of Toronto
R-spondin
Lumibao JC, Okhovat SR, Peck KL et al.
The effect of extracellular matrix on the precision medicine utility of pancreatic cancer patient-derived organoids
From the lab of Dannielle Engle, Salk Institute for Biological Studies

A promising application of organoids, in this case patient-derived organoids (PDOs), is to profile the therapeutic sensitivity of tumors to inform clinical decisions and to stratify patients in clinical trials more effectively. Recently there have been positive results from clinical trials giving a glimmer of the potential impact of these technologies. However, a concern is how reproducible these technologies are, particularly as most still rely on animal-derived basement membrane extracts, where batch variation is a sector-wide concern. This recent publication shows consistent drug responses are observed when different sources of commercial BME were compared. This is reassuring and speaks to how robust these approaches can be.
Ku B, Eisenbarth D, Baek S et al.
PRMT1 promotes pancreatic cancer development and resistance to chemotherapy
From the lab of Dae-Sik Lim, KAIST, Republic of Korea
Marsee A, Ritchie A, Myszczyszyn A et al.
Mass Generation and Long-term Expansion of Hepatobiliary Organoids from Adult Primary Human Hepatocytes
From the lab of Bart Spee, Hubrecht Institute, The Netherlands
Osorio-Vasquez V, Lumibao JC, Peck KL et al.
Identification of molecular and functional subtypes using chronic pancreatitis patient-derived organoid models
From the lab of Dannielle D. Engle, Salk Institute for Biological Studies
Agarwal R, Dittmar T, Beer HD et al.
Human epidermis organotypic cultures, a reproducible system recapitulating the epidermis in vitro
Choi W, Kim YH, Woo SM et al.
Establishment of Patient-Derived Organoids Using Ascitic or Pleural Fluid from Cancer Patients
From the lab of Sun-Young Kong, National Cancer Center, Korea
Feofanov M, Daubner GM, Saltalamacchia A et al.
Discovery and optimization of a guanylhydrazone-based small molecule to replace bFGF for cell culture applications
SCF
Azami T, Theeuwes B, Ton M-LN et al.
STAT3 signalling enhances tissue expansion during postimplantation mouse development
TGF-β
Shin D, Kim CN, Ross J et al.
Thalamocortical organoids enable in vitro modeling of 22q11.2 microdeletion associated with neuropsychiatric disorders
From the lab of Tomasz Nowakowski, University of California, San Francisco

This recent study from David Shin in the lab of Tomasz J. Nowakowski University of California, explores thalamic dysfunction in psychiatric disorders, focusing on the 22q11.2 microdeletion associated with increased risk. They used human pluripotent stem cell-derived organoids to investigate early thalamus development, revealing widespread transcriptional dysregulation and elevated FOXP2 expression in thalamic neurons and glia. From a co-culture model, they found that the microdeletion leads to thalamic axon overgrowth, mediated by FOXP2. These findings suggest dysregulated thalamic development contributes to schizophrenia-related neural phenotypes in 22q11.2 deletion syndrome, offering fascinating insights into the neuropsychiatric disorder’s genetic mechanisms. It will be very interesting to see how human pluripotent stem cell-derived organoids continue to play a fundamental role in psychiatric disorders in the future!
Truszkowski L, Bottini S, Bianchi S et al.
Refined home-brew media for cost-effective, weekend-free hiPSC culture and genetic engineering
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
Used:
- Recombinant human activin A protein (Qk001)
- Recombinant human FGF-2 (145 aa) protein (Qk025)
- Recombinant human FGF-2 (154 aa) protein (Qk027)
- Recombinant human BMP-4 protein (Qk038)
- Recombinant human NRG-1 protein (Qk045)
- Recombinant FGF2-G3 (145 aa) protein (Qk052)
- Recombinant FGF2-G3 (154 aa) protein (Qk053)
- Recombinant human TGF-β3 protein (Qk054)
- Recombinant human TGF-β1 PLUS™ protein (Qk010)

Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
Venkatesan M, Semper C, Skrivergaard S et al.
Recombinant production of growth factors for application in cell culture
From the lab of Alexi Savchenko, University of Toronto
Lyra-Leite DM, Copley RR, Freeman PP et al.
Nutritional requirements of human induced pluripotent stem cells
From the lab of Paul W. Burridge, Northwestern University Feinberg School of Medicine
Beltran-Rendon C, Price CJ, Glen K et al.
Modeling the selective growth advantage of genetically variant human pluripotent stem cells to identify opportunities for manufacturing process control
From the lab of Robert Thomas, Loughborough University

Robert Thomas’s lab at Loughborough University has analyzed growth dynamics between commonly occurring genetically variant hPSCs and their counterpart wild-type cells in culture cells using proprietary computational modelling, allowing the identification of critical process parameters that drive critical quality attributes when genetically variant cells are present within the system This fascinating paper highlights how the system parameters controlling independent growth behavior of wild-type and genetic variant populations are altered when both populations exist within a co-culture environment by introducing an ordinary differential equation (ODE) framework. Findings reveal that variant cells exhibit selective growth and competitive advantage, influencing the behavior of wild-type cells, particularly at higher culture densities. This computational model offers opportunities for defining operational protocols and timely detection of emerging variants, crucial for product release and risk management. It is clear to see the importance as it demonstrates the utility of computational models in understanding complex biological systems and informing manufacturing practices in hPSC-based therapies.
VEGF
Akiyama H, Katayama Y, Shimizu K and Honda H
Engineering a Controlled Cardiac Multilineage Co-Differentiation Process Using Statistical Design of Experiments
From the lab of Hiroyuki Honda, Nagoya University, Japan.