Currency
Recombinant mouse LIF protein (Qk018)
Mouse LIF (murine leukemia inhibitory factor) protein maintains the pluripotency and self-renewal of mouse embryonic and induced pluripotent stem cells.
Qkine recombinant mouse LIF protein is bioactive, animal origin-free and carrier-protein free for highly reproducible results.
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
1000µg will be despatched as 2 x 500µg
Fast and free shipping.
Buy online with secure credit card or purchase order.
For any questions, please email orders@qkine.com
Summary
High purity mouse LIF protein (Uniprot: P09056)
>98%, by SDS-PAGE quantitative densitometry
20 kDa
Expressed in E. coli
Animal origin-free (AOF) and carrier protein-free.
Manufactured in our Cambridge, UK laboratories
Lyophilized from acetonitrile, TFA
Resuspend in 10mM HCl at >100 µg/ml (provided with protein and free of charge), prepare single use aliquots, add carrier protein if desired and store frozen at -20°C or -80°C
Featured applications
Propagation of mouse ES cells
Species similarity:
Human – 97%
Frequently used together
Bioactivity
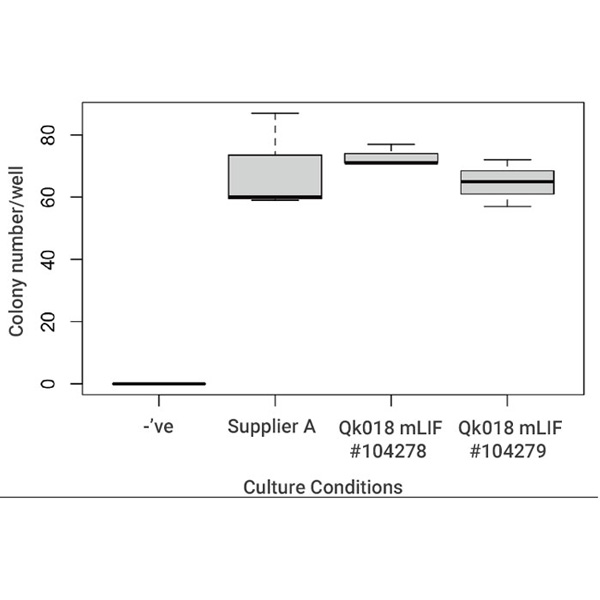
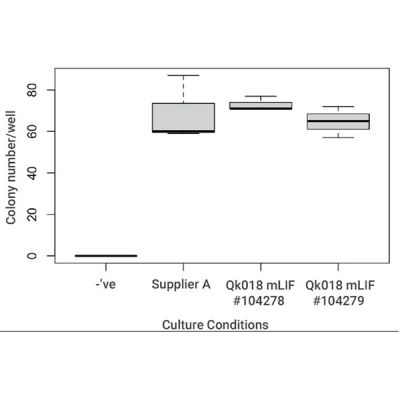
Recombinant mouse LIF protein (animal-free) supports mouse embryonic stem cell colony formation and has been benchmarked against LIF supplement from Supplier A in chemically-defined feeder-free culture. Mouse LIF (Qk018) support mouse ES cell propagation in chemically-defined, feeder-free iPSC culture. Cultures are dissociated to a single cell suspension and plated at very low (clonal) density in defined media containing Qk018 recombinant mouse LIF or mouse LIF supplement from another supplier (Supplier A). The number of colonies that formed was determined after 4-5 days. Data from Qk018 lot #104278 and #104279.
Our thanks to Leitch lab, MRC London Institute of Medical Sciences, Imperial College for mouse ES cell data detailed above and discussion.
Purity
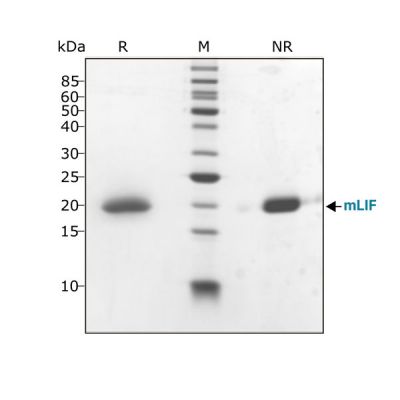
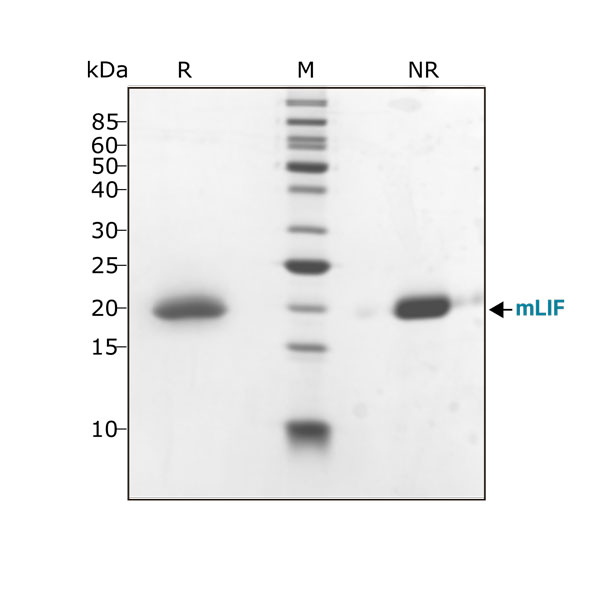
Mouse LIF protein (Qk018) migrates as a single band at 20 kDa in non-reducing (NR) and upon reduction (R) with β-mercaptoethanol. Purified recombinant mouse LIF protein (3 μg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptoethanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250. Data from Qk018 lot #104278.
Further quality assays
Mass spectrometry: single species with expected mass
Endotoxin: <0.005 EU/μg protein (below level of detection)
- Recovery from stock vial: >95%
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
LIF is a pleiotrophic factor that belongs to the IL-6 superfamily of cytokines. Acting through a heterodimeric receptor of LIFR (gp190) and gp130, LIF activates a number of cellular pathways including the JAK/STAT, PI3K and MAPK pathways [1]. LIF signalling is essential for maintenance of murine pluripotency [2,3]. Human LIF can be used for the maintenance of mouse embryonic stem cells, however mouse LIF cannot bind to the human receptor, thus rendering mouse LIF inactive on human cells [4].
Customer & collaborator data
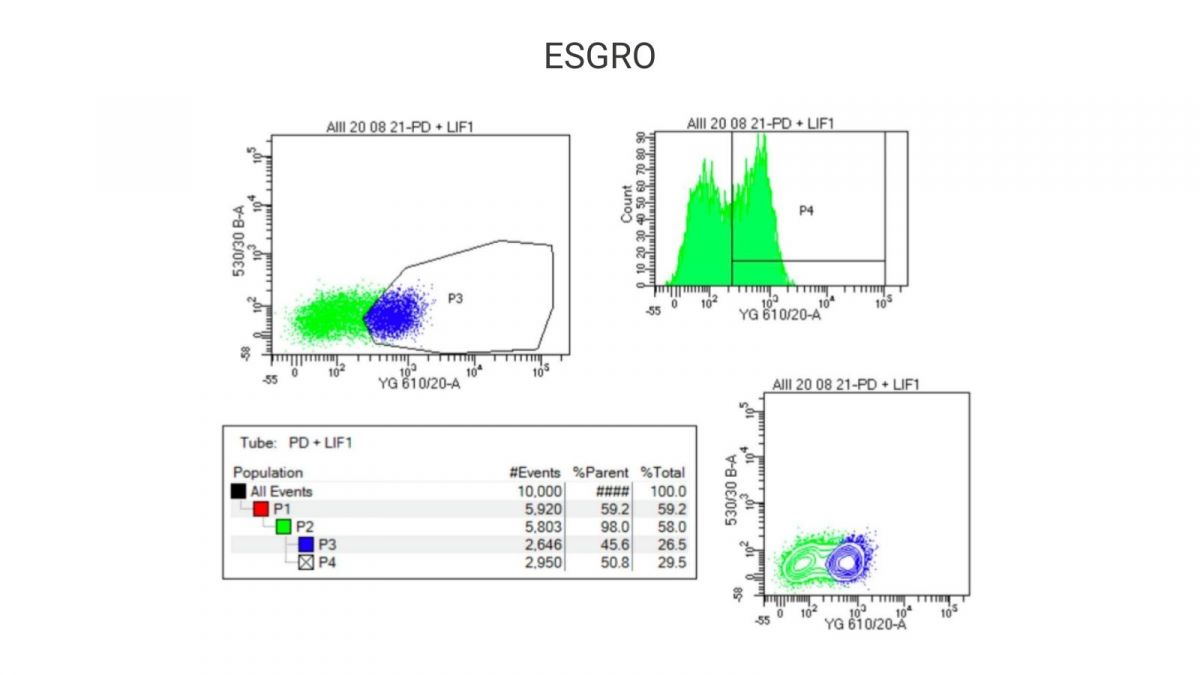
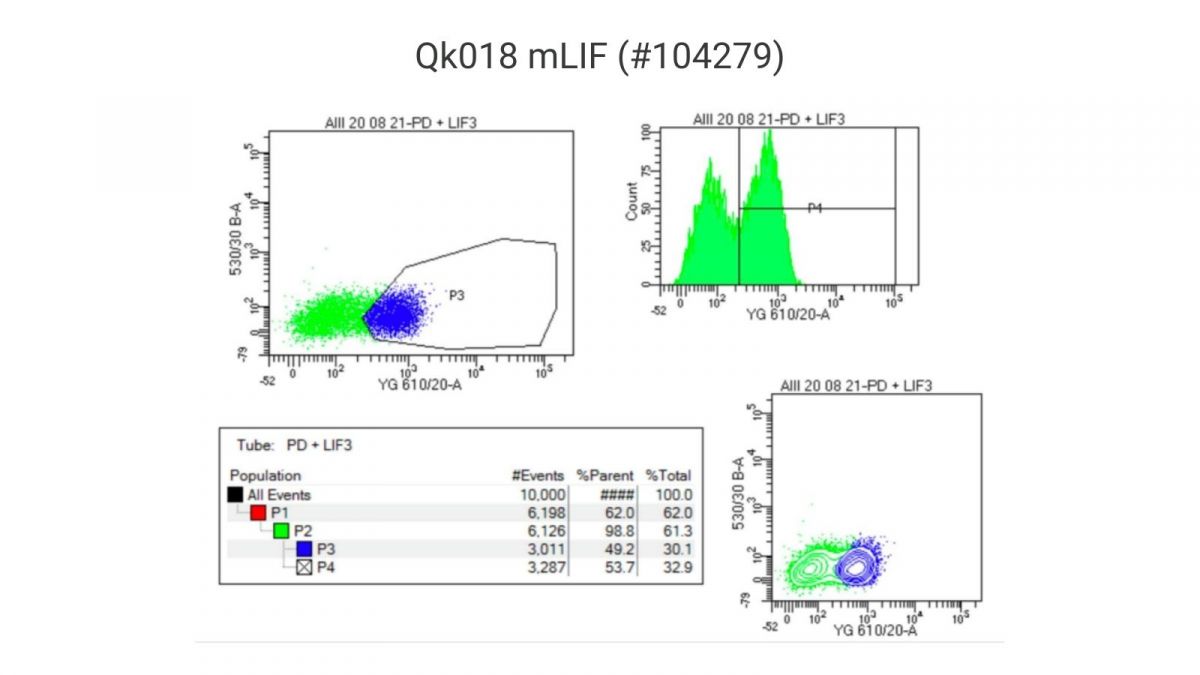
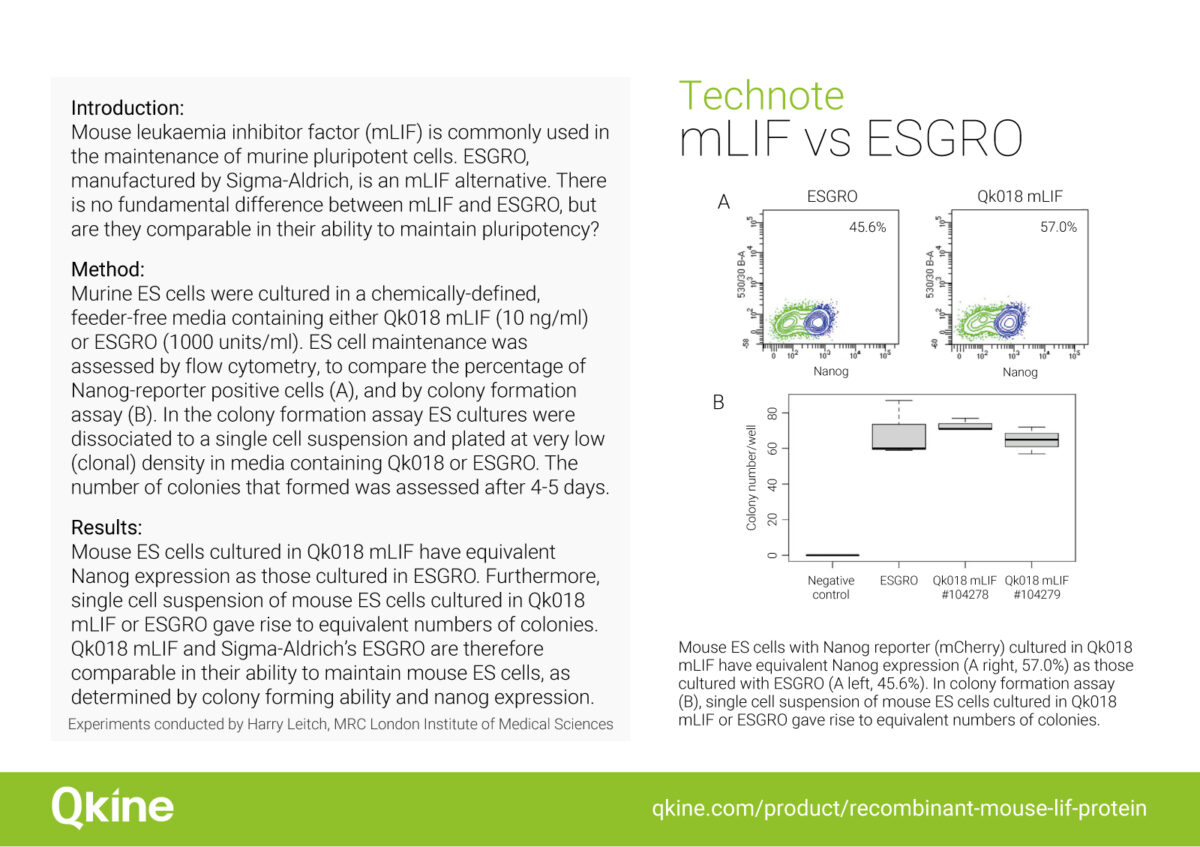
Flow cytometry analysis of Nanog-reporter positive mouse ES cells cultured using Qk018 mouse LIF vs. ESGRO
Flow cytometry was used to compare the percentage of Nanog-reporter positive mouse ES cells cultured in a chemically-defined, feeder-free iPSC culture media containing either Qk018 mouse LIF or ESGRO. Our results show that Nanog expression in mouse ES cells cultured with Qk018 mouse LIF was comparable to, if not potentially better than, ESGRO.
Experiments performed by Harry Leitch (MA MB BChir PhD MRCPCH), MRC London Institute of Medical Sciences, Imperial College.
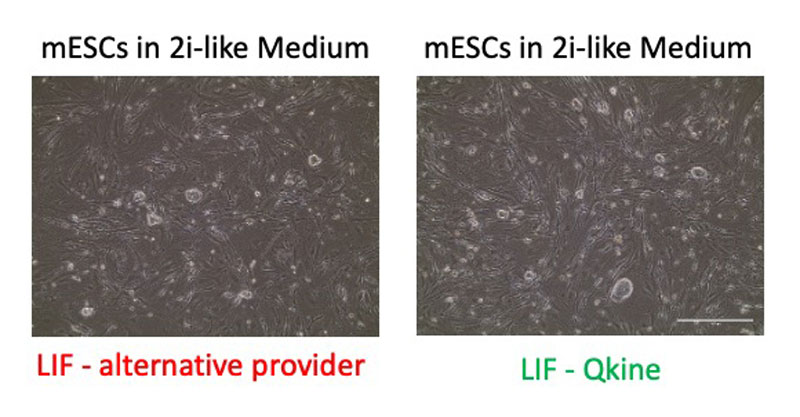
The LIF we tested from Qkine has shown excellent quality in maintaining mouse embryonic stem cells derived from precious samples and maintaining them in a pluripotent state.
Experiments conducted by Dylan Liabeuf.
Publications
Protocol for generating mouse morula-like cells resembling 8- to 16-cell stage embryo cells
In ScienceDirect on 21 June 2024 by Huanhuan Li et al.
CartoCell, a high-content pipeline for 3D image analysis, unveils cell morphology patterns in epithelia
In Cell Reports Methods on 25 September 2023 by Andrés-San Román, J. A. et al.
Neural tube organoids self-organise floorplate through BMP mediated cluster competition
Preprint on 16 July 2023 by Krammer, T. et al.
Negative feedback on Retinoic Acid by Brachyury guides gastruloid symmetry-breaking
Preprint on 4 June 2023 by Hennessy, M. J., Fulton, T., Turner, D. A. & Steventon, B.
Mouse Primordial Germ Cells: In Vitro Culture and Conversion to Pluripotent Stem Cell Lines
In Methods in Molecular Biology on 19 September 2020 by Borkowska, M. & Leitch, H. G.
Distinct Molecular Trajectories Converge to Induce Naïve Pluripotency
In Cell Stem Cell on 15 August 2019 by Stuart, H. T. et al.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.




What others are saying
There are no contributions yet.