Currency
Recombinant human R-spondin 1 protein (Qk006)
R-spondin 1 protein (RSPO1) is the prototypic member of the R-spondin family and is used to potentiate Wnt signaling in many organoid culture systems including intestinal and tumor (cancer) organoid culture. R-spondin 1 is also required for hematopoietic stem cell specification and cancer cell migration and survival.
Recombinant human R-spondin 1 protein (RSPO1) protein is the bioactive domain of human R-spondin 1 comprising the two cysteine-rich furin-like domains of R-spondin 1. Those are necessary and sufficient for Wnt signaling potentiation and are the essential domains for activity in stem cell and organoid culture.
This protein has a molecular weight of 13 kDa and is animal origin-free, carrier protein-free, and tag-free to ensure its purity with exceptional lot-to-lot consistency. Qk006 is suitable to replace R-spondin conditioned media for improved reproducibility in chemically defined organoid culture media.
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
1000µg will be despatched as 2 x 500µg
Fast and free shipping.
Buy online with secure credit card or purchase order.
For any questions, please email orders@qkine.com
Summary
High purity recombinant human R-spondin 1 protein (Uniprot: Q2MKA7)
>98%, by SDS-PAGE quantitative densitometry
13 kDa
Expressed in E. coli
Animal-free (AOF) and carrier protein-free.
Manufactured in our Cambridge, UK laboratories
Lyophilized from acetonitrile, TFA
Resuspend in 10mM HCl at >100 µg/ml (provided with protein and free of charge), prepare single use aliquots, add carrier protein if desired and store frozen at -20°C or -80°C
Featured applications
Pancreatic tumor organoid culture
Maintenance of urine-derived stem cells
Human
Species similarity:
Porcine – 98%
Mouse – 96%
Rat – 96%
Bovine – 94%
R-spondin 1 LR5 protein (Qk031): a specialized form of R-spondin 1 that specifically binds to the LGR5 receptor, developed in the lab of Marc de la Roche (University of Cambridge).
Bioactivity
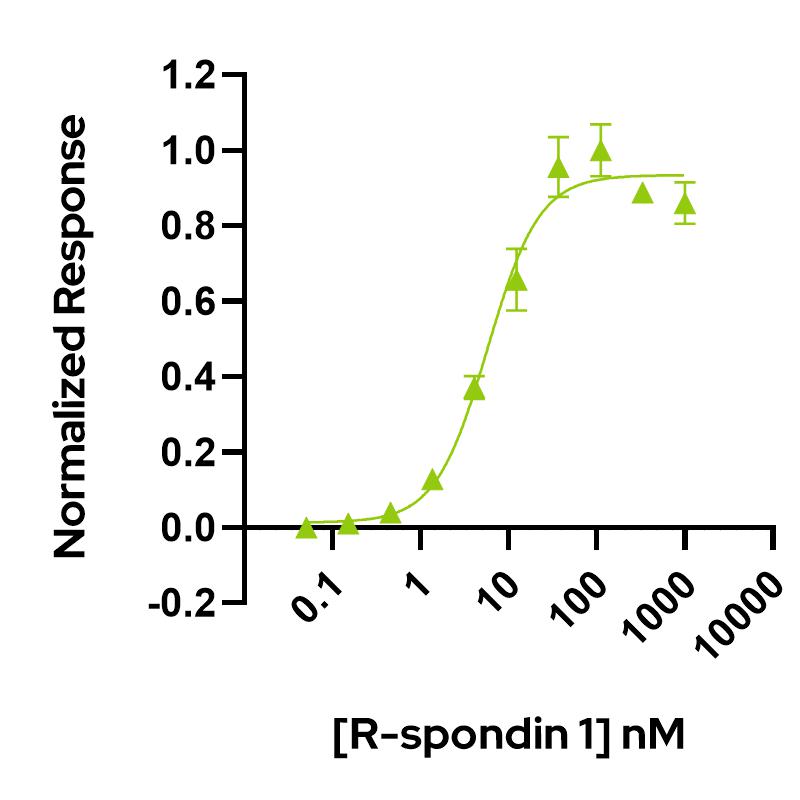
Qk006 activity is determined using the Wnt-responsive firefly luciferase reporter assay as it enhances Wnt-ß catenin signaling in HEK239T cells. HEK293T cells transfected with reporter TOP-FLASH are treated in triplicate with increasing concentration of R-spondin 1 (diluted in DMEM with 0.5 % of FCS), in the presence of Wnt-conditioned media (1:8 dilution). Cells are grown overnight, and luciferase activity is measured and normalized. EC50 = 5.8 nM (75.1 ng/mL). Data from Qk006 lot #104277.
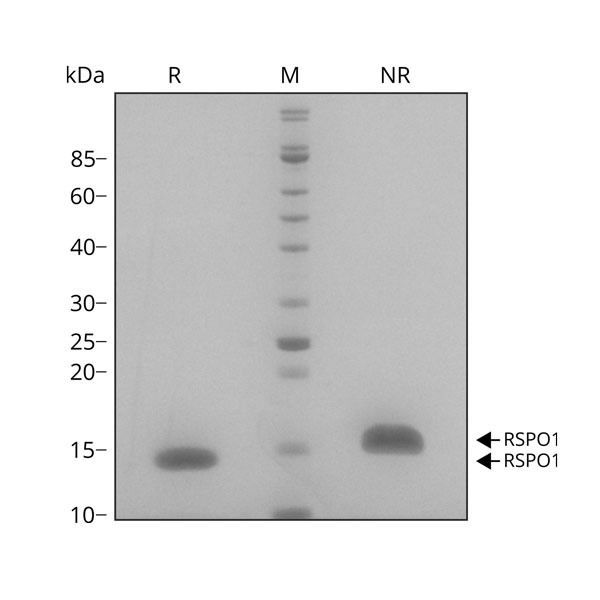
Purity
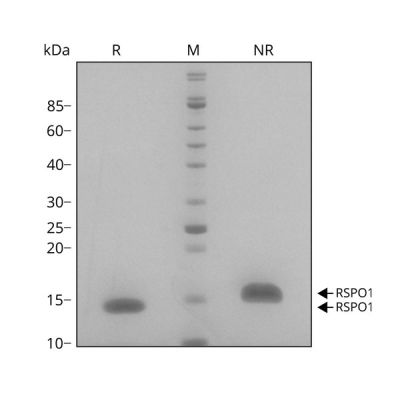
RSPO1 migrates as a single band at 16 kDa in non-reducing (NR) and 13 kDa in reducing (R) conditions. Purified recombinant protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250. Data from Qk006 lot #010.
Further quality assays
Mass spectrometry: single species with expected mass
Analytical reversed-phase: single sharp peak
Endotoxin: <0.005 EU/μg protein (below level of detection)
Recovery from stock vial: >95%
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
R-spondin 1 (RSPO-1) is a secreted protein of the RSPO family that regulates the canonical Wnt signaling pathway [1,2]. The Wnt pathway is a highly conserved signaling pathway that is essential for various cellular processes, including cell proliferation, differentiation, and migration. This growth factor plays a crucial role in embryonic development and patterning, paticularly in the development of the gastrointestinal tract, neural tube, skin, and limbs [3]. It 1 is involved in the maintenance of stem cells and can enhance the self-renewal capacity of stem cells, promoting tissue regeneration and repair [4]. It is also involved in the sex determination and the development and maintenance of the female sex characteristics in particular the differentiation and maintenance of the ovarian tissues [5].
RSPO1 proteins are generally characterized by a core domain known as the thrombospondin type 1 (TSP1) domain, which is responsible for their signaling activity. This protein is comprised of 263 amino acids and structured as two cysteine-rich, furin-like domains [3,6]. It acts as an agonist (activator) of the Wnt signaling pathway by binding to the leucine-rich repeat-containing G-protein coupled receptors (LGR) 4-6, leading to the stabilization of β-catenin, a key component of the pathway [7].
In cell culture, this recombinant protein is used extensively for the maintenance of self-renewal and expansion of stem cells. It is also used for the culture of intestinal, hematopoietic, and epithelial stem cells [2,7,8]. Also, the culture of stem cell-derived organoids such as intestinal, pancreas liver, colon, and stomach organoids benefit from the inclusion of RSPO1 in the media [7,9,10].
Dysregulation of the Wnt signaling pathway, including the overexpression of R-spondin 1, has been associated with tumor growth and progression in various cancers such as colon and ovarian cancer [11,12]. Research is ongoing on the role of R-spondin 1 in various biological processes and its potential as a therapeutic target for cancer treatment. Given its role in stem cell maintenance and tissue regeneration, RSPO1 and related proteins have also been explored as potential therapeutic targets for regenerative medicine.
Customer & collaborator data
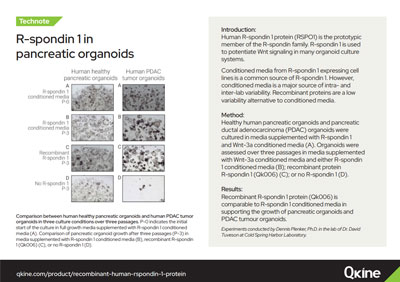
Qkine recombinant human R-spondin 1 protein replaces conditioned media in pancreatic tumor organoid culture – data from Tuveson Laboratory, Cold Spring Harbor Lab
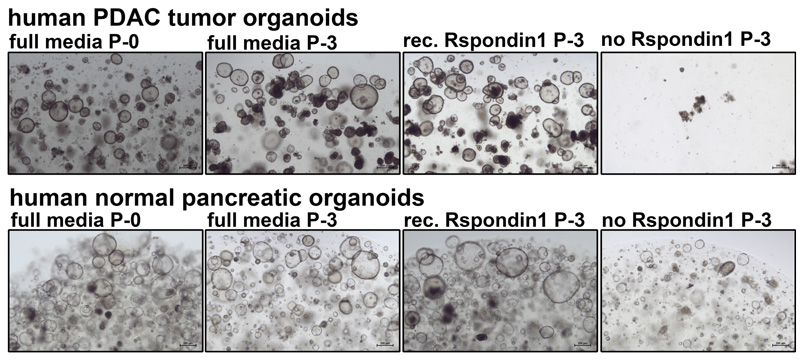
Comparison of pancreatic organoid growth over three passages. P-0 indicates the initial start of the culture in full growth media supplemented with Wnt3a and R-spondin 1-conditioned media. ‘Rec. R-spondin 1’ culture conditions were created by depleting full growth media of RSPO1-conditioned media and supplementing it with Qk006. ‘No R-spondin 1’ culture conditions were created by depleting full growth media of RSPO1-conditioned media but not subsequently supplementing the media with an alternative source of R-spondin 1. No differences in organoid growth have been observed when using recombinant R-spondin 1 instead of RSPO1-conditioned media. Experiments have been conducted by Dennis Plenker, Ph.D. in the lab of Dr David Tuveson at Cold Spring Harbor Laboratory.
Qkine R-spondin 1 treatment on urine-derived stem cells maintains cellular morphology and nuclear expression of the nephron progenitor marker, SIX2
Previous research suggests that the removal of R-spondin 1 from the culture media of urine-derived stem cells leads to loss of kidney progenitor molecules in mice. Therefore, it was hypothesized that the addition of R-spondin 1 to the culture media would result in maintenance of SIX2 progenitor molecules and thus maintenance of the undifferentiated state.
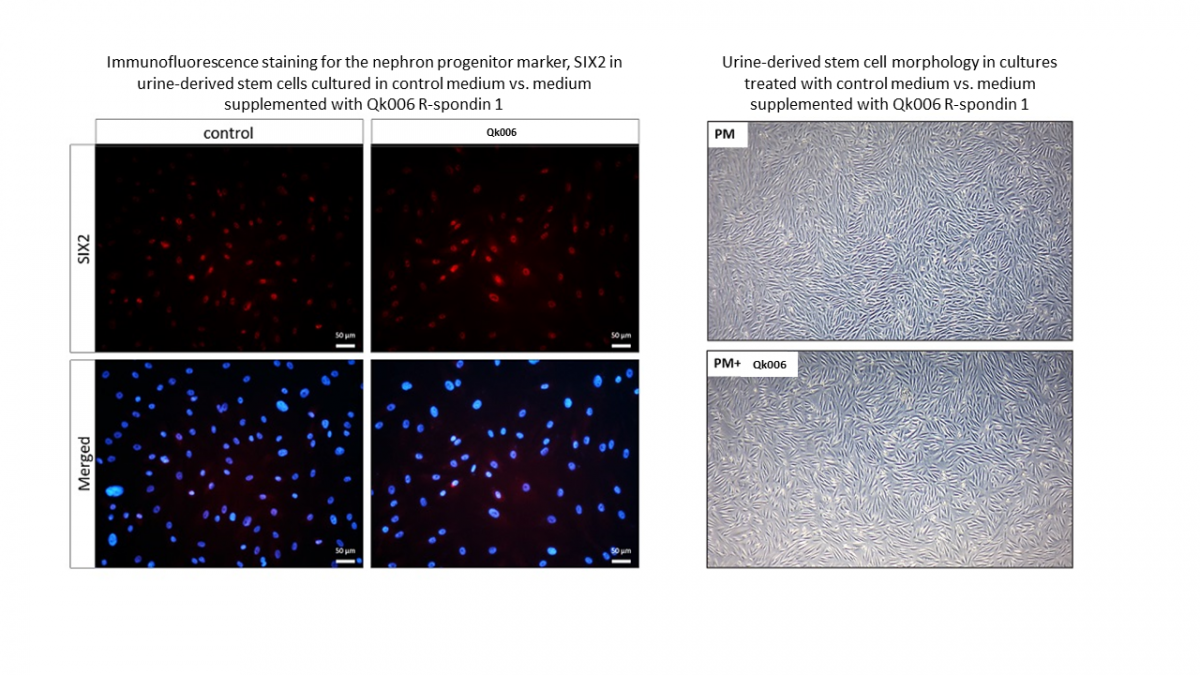
Urine-derived stem cells were treated with Qk006 R-spondin 1 at a concentration of 200 ng/ml for 5 days. The cells were then fixed and stained for SIX2.
Recombinant human R-spondin 1 protein (Qk006) maintains urine-derived stem cell morphology. Urine-derived stem cells typically have a small, rice grain-like morphology and express nuclear SIX2. Comparing the morphology of untreated vs treated cells showed that cells treated with Qk006 R-spondin 1 had a similar morphology to the control, indicating that R-spondin 1 treatment maintains the undifferentiated state.
Recombinant human R-spondin 1 protein (Qk006) maintains nuclear SIX2 expression in urine-derived stem cells. In urine-derived stem cells, SIX2 expression is predominately found in the cell nuclei, as shown in the untreated control. Cytoplasmatic expression of SIX2 was also observed, which indicates cell differentiation. Addition of Qk006 R-spondin 1 maintained nuclear SIX2 expression (as shown by immunofluorescence staining for SIX2) and therefore appears to keep the urine-derived stem cells in an undifferentiated state.
Experiments have been conducted by Lisa Nguyen (MSc) and team in the laboratory of Professor James Adjaye at the Institute for Stem Cell Research and Regenerative Medicine, University of Dusseldorf.
Publications
PRMT1 promotes pancreatic cancer development and resistance to chemotherapy
In Cells Reports Medicine on 8 March 2024 by Ku, B., Eisenbarth, D., et al.
The effect of extracellular matrix on the precision medicine utility of pancreatic cancer patient–derived organoids.
In JCI Insight on 5 December 2023 by Lumibao, J.C., Okhovat, S.R, et al.
Establishment of Patient-Derived Organoids Using Ascitic or Pleural Fluid from Cancer Patients
In Cancer Research and Treatment on 12 June 2023 by Choi, W et al.
Human epidermis organotypic cultures, a reproducible system recapitulating the epidermis in vitro
In Experimental Dermatology on 28 April 2023 by Agarwal, R. et al.
FAQ
R-spondin 1 regulates Wnt signaling and β-Catenin stabilization. Therefore, the functions of R-spondin 1 are diverse and are associated with cell proliferation and differentiation. It maintains the self-renewal capacity of stem cells and is involved in the embryonic development especially the development of the female reproductive system, gastrointestinal track, and neural tube.
The Wnt signaling pathway is involved in in various biological processes. It is involved in the cell fate specification, embryonic development, stem cell maintenance, tissue regeneration, synaptic function, immune response, cancer progression, and cell migration.
The molecular weight is 13 kDa.
The RSPO1 gene provides instructions for producing the R-spondin 1 protein.
The RSPO1 gene is located at 1q41.2.
It is a growth factor supplemented to cell culture media. It is used for the culture of induced pluripotent stem cells, embryonic stem cells, epithelial stem cells, and organoids such as intestinal and colon organoids
The size of the R-spondin proteins, including R-spondin 1 (RSPO1), can vary slightly depending on the specific isoform and the species being considered. In humans, RSPO1 is approximatively 263 amino acids in length with a molecular weight of 13-29 kDa.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.


What others are saying
There are no contributions yet.