FAQs
- Handling & storage
- Ordering & shipping
- Protein R&D and biochemistry
- Protein testing & quality
- Regulatory
Qkine uses freeze drying to remove water and other solvents from our growth factors to ensure the preservation of activity, improve solubility, and enhance protein stability during shipping and storage. This is achieved by freezing the target proteins with selected excipients and cryoprotectants and allowing the frozen solvents to sublimate under vacuum.
Our freeze-dried growth factors will usually form a well-formed porous structure (the cake) which retains the physical size of the volume of liquid when frozen i.e. a 25 µg vial will contain a cake of roughly 25 µl. In some cases, our growth factors are formulated to be stable in the absence of bulking agents, such as mannitol, and tend to undergo a collapse of the porous structure of the cake during lyophilization. This results in a powdery/speckled appearance without affecting the stability or activity of the proteins.
The appearance of the lyophilized product depends on the exact excipients used and the resulting glass transition temperature, but we would routinely expect to see the following types of structures for our products:
- The product appears as a white powder speckled up to the height of the initial liquid. This is due to a lack of a bulking agent resulting in a cosmetic cake collapse during lyophilization.
Typical for products lyophilized from Tris, HEPES/NaCl, PBS, Tris/NaCl, HEPES/NaCl/CyS or Tris/NaCl/CyS
- The product appears as a visible cake of roughly equal volume to the initial volume of liquid. Due to the lack of a bulking agent some shrinkage may occur when air is reintroduced after lyophilization resulting in separation of the cake from the tube walls.
Typical for products lyophilized from acetonitrile/TFA
- The product appears as a well-formed visible cake of roughly equal volume to the initial volume of liquid.
Typical for products lyophilized from Tris/mannitol, Tris/NaCl/CyS/mannitol or acetonitrile/TFA/mannitol
- The product appears as a well-formed visible cake of roughly equal volume to the initial volume of liquid. Some puffing of the cake may be visible due to expansion of air bubbles during sublimation without affecting protein stability.
Typical for products lyophilized from HEPES/NaCl/mannitol
- The product may appear translucent
With Qkine’s continuous improvement strategy, our freeze-drying process has developed and evolved. It is normal for products dried under the previous lyophilization process to appear translucent.
Qkine has implemented an ISO9001:2015 compliant quality management system encompassing its entire R&D, manufacturing, and operations functions. Embedding this internationally recognized standard demonstrates a commitment to consistently meet and exceed customer expectations and applicable statutory and regulatory requirements. UKAS accredited BSI, respected globally, was selected to certify the quality management system giving enhanced confidence in the assessment of best practice.
ISO9001: 2015 Certificate number: FS 747253
Location: 1 Murdoch House, Garlic Row, Cambridge, CB5 8HW, UK


Address: 1 Murdoch House, Garlic Row, Cambridge, CB5 8HW, UK
Tel No: +44 (0) 1223 491486
Company registration no: 10470662
VAT No: GB265464484
A wide range of analytical techniques can be used to measure the various properties of a protein, such as the size, charge, amino acid composition and hydrophobicity. Confirmation of the purity of growth factors by several varied techniques ensures reliability of results. When shopping for growth factors look for products where purity has been validated by multiple methods. Some common purity assessment methods are discussed below.
What is an SDS-PAGE gel and how do you interpret it? What are reducing vs non-reducing conditions?
SDS-PAGE (sodium dodecyl sulfate – polyacrylamide gel electrophoresis) is a method that separates protein by its mass. SDS is an ionic detergent that denatures and binds to proteins to make them uniformly negatively charged. This means that when an electronic current is applied to a polyacrylamide gel, the SDS bound proteins will migrate down the gel towards the positively charged electrode, separated by size alone.
Proteins can be reduced before being run on an SDS-PAGE gel. In reducing conditions β-mercaptoethanol (β-ME or 2-ME) or dithiothreitol (DTT) is added; this reduces the disulphide bridges in proteins so that when they are run on the gel, they are better separated by size. Many of our growth factors are disulfide-linked dimers and so running non-reduced proteins on the same gel as reduced proteins allows confirmation of the correct dimeric state.
What should I look for from mass spectrometry analysis?
Mass spectrometry analysis is used to confirm the molecular mass of the intact protein and to reveal any heterogeneity that would not be evident from SDS-PAGE analysis. The resultant mass is compared with calculated mass of the protein with the assumption that all the cysteines are disulfide-linked. Multiple peaks represent different charge states of the protein.
What does analytical reversed-phase chromatography show?
Analytical reversed-phase chromatography is used to assess protein purity and structural homogeneity. Homogeneity is judged by the absence of multiple peaks and by the symmetry of the main peak. In these traces look for a single sharp peak; this indicates a single species is present in the sample with no contamination.
Endotoxin levels; what problems can endotoxins cause? What level of endotoxin contamination is acceptable?
Endotoxins are small hydrophobic lipopolysaccharide molecules, a toxic substance found in the outer cell membrane of gram-negative bacteria. Endotoxins are shed by bacteria during their cell death or when they are actively growing and dividing. Endotoxins can affect the growth or performance of cell cultures and are a major source of experimental variation. Endotoxins have a very high heat stability meaning they cannot be destroyed with regular decontamination methods such as autoclaving. Endotoxins are also hydrophobic, and consequently have a strong affinity for other hydrophobic materials such as lab plastics. Endotoxins can be avoided by cleanliness and proper lab techniques to keep endotoxin levels in a lab at bay. At Qkine, we achieve this with good aseptic technique, filter sterilization of buffers used during manufacturing processes and rigorous purification column cleaning protocols. This will minimize introduction of endotoxins into any manufacturing/research processes.
To determine the presence of endotoxins, a limulus amebocyte lysate (LAL) assay is used. This assay can detect as little as 0.01 endotoxin units (EU)/mL. One EU equals approximately 0.1 to 0.2 ng endotoxin/mL of solution. Current industry standards require endotoxin levels in growth factor lots to be less than 0.5 EU/mL, however the lower the better, and companies such as Qkine impose internal standards of <0.1 EU per µg protein.
It is very hard to determine the activity of a growth factor as they have a large range of potential effects. A luciferase reporter assay is used to quantify the response of transfected cells to a growth factor by assaying the expression of luciferase (the reporter gene). As the biological activity of a growth factor is measured by its effect on a defined cell type in standardised conditions, it may not accurately represent your own culture conditions, however it will provide confirmation that the growth factor is able to elicit a biological response.
To determine this bioactivity, a vector containing a firefly luciferase gene under the control of a growth factor appropriate regulatory region is introduced into cells, such as HEK293Ts. These are then incubated with a dilution series of the growth factor. After a defined incubation period, the bioactivity is measured by quantifying the enzymatic activity of luciferase. This involves lysing the cells and adding the luciferase substrate, luciferin. The amount of light emitted from the reaction is directly proportional to the amount of luciferase in the sample and consequently can be used as a measure of the level of response the growth factor imparts on the reporter gene. In our QC experiments, we also quantify Renilla luciferase which is expressed constitutively. This acts as a normalization control for transfection efficiency and the cell number in each well. The growth factor response can then be expressed as a firefly/Renilla ratio. Plotting concentration vs. F/R ratio allows an EC50 (half-maximal effective concentration) to be calculated.
The EC50 is the concentration of the growth factor which gives 50% of the maximum effect on the given cell population
When shopping for growth factors, you want to confirm that the protein has been established to be consistently bioactive across multiple lots, ensuring each lot you receive will be equally as active as that reported. Our quality control process includes validation with a previous lot to ensure excellent lot-to-lot consistency (e.g. Figure 1). This consistency is essential when treating cells with growth factors to prevent experiment variability. The activity of a growth factor can differ significantly depending on experimental conditions. This means that comparisons between growth factors from different suppliers are tricky to make. At Qkine, we have validated most of our growth factors against other suppliers’ proteins under our experimental conditions to ensure that we are providing you with products that are as good as, or better than, those you may have used previously.
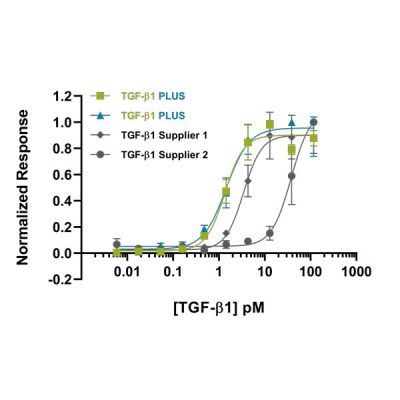
Growth factors can be synthesised in prokaryotic cells, most commonly E. coli, or eukaryotic cells, using cell lines such as human embryonic kidney (HEK) 293 cells or Chinese hamster ovary (CHO) cells. There are benefits to each of these cell sources. Proteins produced from prokaryotic sources generally have less lot-to-lot variability and higher purity than eukaryotic systems. They have the additional benefit of being animal-free, eliminating the risks of contaminating growth factors, viruses, prions and other animal-derived ingredients. This production method also reduces the need for foetal bovine serum (FBS), use of which is an ethical concern for many researchers. However, when it comes to complex full length mammalian proteins prokaryotic systems can fall short; protein refolding and post translational modification can differ from eukaryotic systems which can affect protein solubility and activity. For growth factors from both prokaryotic and eukaryotic sources, please be sure to check the bioactivity data before you purchase.
Innovations in growth factor production are allowing an increasing number of proteins to be produced using prokaryotic systems, and they benefit from being animal-free, while maintaining correct refolding and activity. Qkine is leading this charge with products including; TGF-β1 PLUS, the first entirely animal-free TGF-β1, and bioactive fragments of human R-spondin 1 and R-spondin 3.
Adding a carrier protein increases protein stability, extends shelf life, allows the protein to be stored at more dilute concentrations, and helps avoid the product sticking to the walls of the vial. Consequently, adding carrier proteins can be very helpful. However, most carrier proteins are animal-derived so for animal-free and sensitive applications and assays for which BSA might interfere with the assay, carrier-free proteins are the way to go. If compatible with your work, you can add your own carrier proteins, such as BSA, HSA or gelatin when you make aliquots. Adding your own carrier allows you to select an application-compatible carrier. We recommend always using a high-purity carrier protein.
Growth factor shelf life is influenced by many factors, including storage state, buffer ingredients, storage temperature and the stability of the protein itself. Generally speaking, growth factors are most stable when lyophilized. Our lyophilized proteins have been verified to be stable at room temperature for 3 days, or frozen for up to 2 years (either at -20 oC or -80 oC).
Once reconstituted, recombinant proteins can be stored at 4oC for 1-2 weeks, however we recommend freezing single use aliquots and defrosting fresh each time to ensure maximum protein stability and activity. Reconstituted proteins can be stored frozen for up to 1 year. When freezing reconstituted growth factors prepare single use aliquots. Every freeze-thaw cycle may cause some denaturation of the protein so repeated freeze-thaw cycles should be avoided. Always check the product sheet for freezing recommendations, but generally -20°C is suitable for short-term storage and -80°C for long term storage.
Always check the manufacturer’s instructions for how to reconstitute your lyophilized proteins (ours is here) and the product datasheet, but here are some general useful tips:
- Centrifuge before opening. During transportation the lyophilized protein can move around and may become lodged in the cap or on the sides of the vial. Before opening the lid, centrifuge to collect all the protein at the bottom of the vial.
- Dissolve in the recommended solution. Many factors affect protein solubility including pH and ionic strength. Reconstitution in the recommended buffer will minimize loss of protein due to precipitation or adsorption onto plastic. For some proteins, e.g. TGF-β superfamily proteins, acid reconstitution is essential as the molecule is hydrophobic and insoluble at neutral pH. For these proteins it is also recommended to store the protein at low pH in order to maintaining the correct disulfide structure of the protein (the low pH minimizes disulfide bond exchange reactions).
- Do not vortex! When reconstituting, do not vortex as the protein will not appreciate the shear stress or air. Simply let the sample sit for a few minutes with the solubilization buffer at room temperature and gently agitate or pipette up and down (avoid foaming).
- Reconstituting within the recommended concentration range will benefit protein stability and aid maximum recovery. Keeping proteins at concentrations outside of this range may result in unstable protein conditions and activity decline. Attempting resuspension at a concentration above the recommended range may also exceed the maximum dissolved concentration of the protein, meaning some protein will not be dissolved.
Lyophilized (freeze dried) proteins are considerably more stable than proteins in solution, consequently many proteins ship in lyophilized forms. This extends the time and temperature range at which the proteins can be shipped while maintaining the activity of the protein – good for the environment and your experiments. Lyophilized proteins are generally stable at room temperature for one month (we verify stability over a standard 3-day shipping cycle), or frozen at -20 oC or -80 oC for 2 years. However, on occasion lyophilization can cause partial loss of activity and damage protein structure, so some proteins are supplied in buffer.
We accept the following currencies via our online store:
- GBP: Pound sterling (£)
- EUR: Euro (€)
- USD: United States (US) dollar (US$)
To change the currency displayed on our website, simply click ‘currency’ on the top navigation bar and select your local or preferred currency from the dropdown list.
The EMA/410/01-Rev3 regulation is not directly applicable to our products as it only applies to medicinal products and the use of animal-derived products as raw materials, which we do not use in any of our manufacturing processes. However, for peace of mind, we are in the process of confirming that everything we use both directly and indirectly for manufacturing our products is compliant with the regulation.
We have compared our lyophilized proteins incubated at 37 °C for 3 days with those stored at -80 °C and found no difference in their bioactivity (EC50) using our standard assays. Therefore, as part of our commitment to the environment and supporting more eco-friendly packaging, we send all of our lyophilized proteins at room temperature, avoiding the need for dry ice, ice packs and gels.
Please order online (where you can upload a PO), email orders@qkine.com or phone +44 (0) 1223 491486 / USA toll free 1-866 877 2185.
Our team are always happy to help and if you need reserved stock from batches you have validated, bulk orders or any further information, please contact us by phone or email (orders@qkine.com)
Every effort is made to ensure samples are sterile; however, we recommend sterile filtering after dilution in media or the final working solution.
TGF beta family proteins and other growth factors can be very poorly soluble in physiological solutions. Please follow the handling guidance for lyophilized cytokines below to minimize loss of protein due to precipitation or adsorption to plastic. We advise storing the recombinant protein at very low pH before dilution in cell culture media or final working solutions. Low pH will also assist in maintaining the correct disulfide structure of the protein by minimizing disulfide bond exchange reactions.
- Resuspension in physiological buffers may cause precipitation of stock solutions, hence we recommend dissolving our lyophilized cytokines in 10 mM HCl (1:1000 dilution of concentrated HCl) while keeping the protein concentration at 50 µg/ml or above, in order to avoid loss by adsorption to plasticware.
- To ensure you recover all of the protein, let the sample sit for a few minutes with the solubilization buffer at room temperature and pipette gently up and down (avoid foaming).
- Rinse the tube with some more 10 mM HCl and pool with the rest.
- The protein is tolerant of some freeze and thaw cycles, but as always with proteins, it is better to aliquot and store frozen.
- Our proteins are supplied carrier-protein free. If compatible with your work, add carrier protein of your choice such as BSA, HSA or gelatin to further minimize loss by adsorption.
- Store in -80°C for long term storage. -20°C for short-term.
Every effort is made to ensure samples are sterile; however, we recommend sterile filtering after dilution in media or the final working solution.
Contact us
Our scientists and operation team are available to answer any queries you have.
Fill in the form below, and a member of our team will get back to you shortly.
