Currency
Recombinant human/mouse/rat/bovine/porcine Activin A protein (Qk001)
Activin A is a TGF–β family growth factor regulating embryonic development, cell proliferation, differentiation, and immune responses. Activin A is frequently used to maintain pluripotency in induced pluripotent and embryonic stem cell cultures. It is also used in many stem cell differentiation protocols, including endoderm lineage differentiation and further maturation into hepatocyte and pancreatic cells.
Recombinant human/mouse/rat/bovine/porcine activin A protein is a high-purity mature bioactive dimer of 26 kDa. It is animal origin-free, carrier protein-free, and tag-free to ensure its purity with exceptional lot-to-lot consistency. This protein has been rigorously benchmarked against other commercial sources and extensively validated for highly reproducible stem cell culture. Qkine founder Marko Hyvönen developed the protocol for making high-purity activin A, and this protein is used at the Cambridge Stem Cell Institute.
High purity mature bioactive dimer. Animal-free (AOF) and carrier-protein free (CF). Rigorously benchmarked against other commercial sources.
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
1000µg will be despatched as 2 x 500µg
Fast and free shipping.
Buy online with secure credit card or purchase order.
For any questions, please email orders@qkine.com
Summary
Bioactive mature domain of recombinant human activin A protein, residues 311-426, (Uniprot: P08476)
>98%, by SDS-PAGE quantitative densitometry
26 kDa (dimer)
Expressed in E. coli
Animal origin-free (AOF) and carrier protein-free.
Manufactured in our Cambridge, UK laboratories
Lyophilized from acetonitrile, TFA
Resuspend in 10mM HCl at >100 µg/ml (provided with protein and free of charge), prepare single use aliquots, add carrier protein if desired and store frozen at -20°C or -80°C
Featured applications
Induced pluripotent and embryonic stem cell differentiation and maintenance
Regulated by the high-affinity inhibitor, follistatin1
human, mouse, rat, bovine, porcine
Frequently used together
Follistatin resistant activin A (Qk035): a specialized form of that is resistant to follistatin feedback, developed in the lab of Marko Hyvönen (University of Cambridge)
Activin a PLUS (Qk005): optimised biologically active truncation of the mature domain of human Activin A protein, developed by Qkine.
Bioactivity
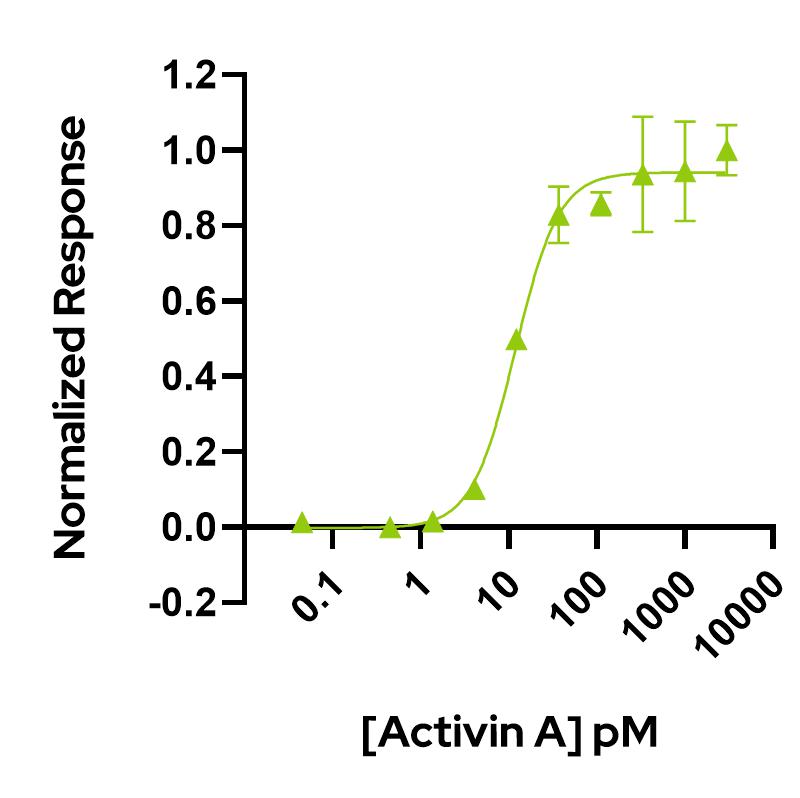
Activin A activity is determined using the activin-responsive firefly luciferase reporter assay in stably transfected HEK293T cells. Cells are treated in triplicate with a serial dilution of Activin A. Firefly luciferase activity is measured and normalized. EC50 = 11.9 pM (309.4 pg/mL). Data from Qk001 lot #104276.
Purity
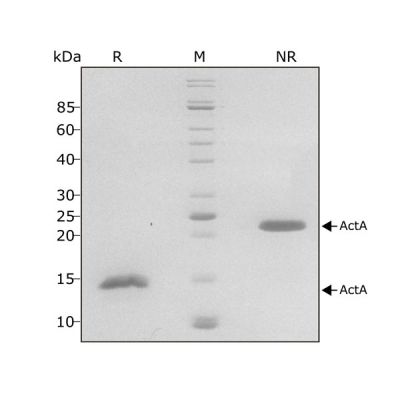
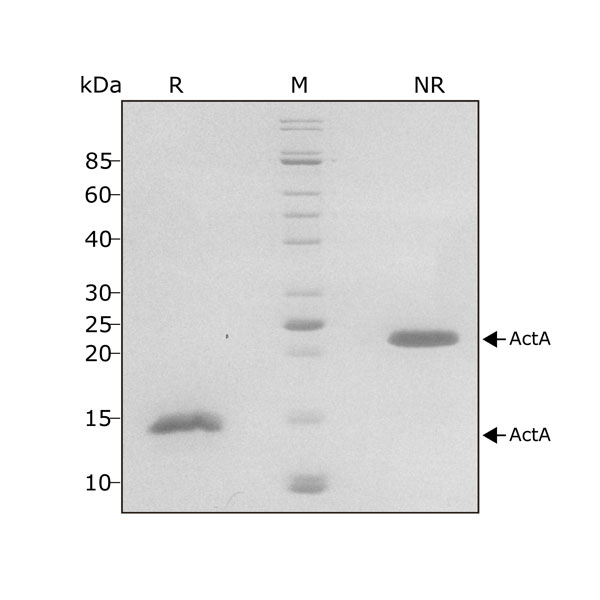
Activin A migrates as a single band at 24 kDa in non-reducing (NR) and 13 kDa as a single monomeric species upon reduction (R). No contaminating protein bands are visible. Purified recombinant protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250. Data from Qk001 lot #011.
Further quality assays
Mass spectrometry: single species with expected mass
Analytical reversed-phase: single sharp peak
Endotoxin: <0.005 EU/μg protein (below level of detection)
Recovery from stock vial: >95%
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
Activin A is a member of the belongs to the transforming growth factor beta (TGF-β) superfamily TGF-β family of growth factors [1]–[4]. It was first identified for its ability to stimulate the release of follicle-stimulating hormone (FSH) from the pituitary gland. It was later recognized as a multifunctional protein with diverse cellular effects [5], [6]. It plays crucial physiological roles in regulating embryonic development, cell proliferation, and differentiation [6], [7]. It promotes the patterning and differentiation of various organs, including the development of the mesoderm, neural, and reproductive systems. It is also involved in maintaining homeostasis, regulating immune responses, and wound healing [8]. Impaired activin A signaling has been associated with various pathological conditions, including cancer, inflammation, and fibrosis [9], [10]. As activin A can contribute to disease progression and severity, it is a growing area of research for promising therapeutic targets.
Activins are disulfide-linked homo- and heterodimers of four inhibin β chains [6]. The best-characterized are activin A and activin B, homodimers of inhibin βA and inhibin βB, respectively. Like all other members of the TGF-β family, activins are synthesized as significant precursors consisting of an N-terminal signal peptide, a pro-domain of 250–350 residues, and a highly conserved mature domain. The pro-domain, which is cleaved off in the mature protein, has essential roles in the biosynthesis, stabilization, transportation and signaling of the growth factors in the body [11]. Activin A binds to activin type I (ALK4 or ALK7) and type II (ActRIIA or ActRIIB) receptors [6], [12]. The type II receptor phosphorylates the type I receptor upon ligand binding, initiating downstream signaling cascades, mainly through the SMAD family of proteins. Activated SMAD complexes translocate into the nucleus, regulating the transcription of target genes involved in various cellular processes. In vivo, the high-affinity inhibitor follistatin and inhibins tightly regulate activin A activity in a feedback loop [13], [14]. Follistatin is secreted into the media during stem cell culture. However, the impact on the efficiency of stem cell differentiation and cellular homogeneity has not been studied closely (see this discussion for more information).
Activin A is frequently used for the maintenance of pluripotency of human induced pluripotent stem cells and human embryonic stem cell lines along with fibroblast growth factor 2 (FGF-2) [2], [4]. Activin A is also used in various stem cell differentiation protocols. It directs the differentiation into definitive endoderm, precursor to different cell types such as pancreatic and liver cells [15]–[18]. Activin A also promotes neural precursor cells and drives astrocytic differentiation with Ciliary neurotrophic factor (CNTF) and Glial cell line-derived neurotrophic factor (GDNF) [19]. Moreover, Activin A is also involved in mesodermal differentiation to derive muscle, bone, and blood cells. Finally, activin A is often used for the development and maintenance of organoids [18], [20], [21]. Qkine recombinant activin A protein has been extensively validated and benchmarked with other suppliers’ proteins in stem cell culture and assays. You can view the results of this analysis and a commentary by Qkine’s founder, Marko Hyvönen.
[1] E. Piek, C. H. Heldin, and P. Ten Dijke, ‘Specificity, diversity, and regulation in TGF-beta superfamily signaling’, FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol., vol. 13, no. 15, pp. 2105–2124, Dec. 1999.
[2] S. Pauklin and L. Vallier, ‘Activin/Nodal signalling in stem cells’, Development, vol. 142, no. 4, pp. 607–619, Feb. 2015, doi: 10.1242/dev.091769.
[3] J. J. Lee and H. F. Rosenberg, Eds., ‘Chapter 12 – Eosinophil-Mediated Tissue Remodeling and Fibrosis’, in Eosinophils in Health and Disease, Boston: Academic Press, 2013, pp. 391–429. doi: 10.1016/B978-0-12-394385-9.00012-2.
[4] L. Vallier, M. Alexander, and R. A. Pedersen, ‘Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells’, J. Cell Sci., vol. 118, no. 19, pp. 4495–4509, Oct. 2005, doi: 10.1242/jcs.02553.
[5] R. S. Carroll, P. M. Kowash, J. A. Lofgren, R. H. Schwall, and W. W. Chin, ‘In vivo regulation of FSH synthesis by inhibin and activin’, Endocrinology, vol. 129, no. 6, pp. 3299–3304, Dec. 1991, doi: 10.1210/endo-129-6-3299.
[6] Y. Xia and A. L. Schneyer, ‘The Biology Of Activin: Recent Advances In Structure, Regulation And Function’, J. Endocrinol., vol. 202, no. 1, pp. 1–12, Jul. 2009, doi: 10.1677/JOE-08-0549.
[7] S. Sulzbacher, I. S. Schroeder, T. T. Truong, and A. M. Wobus, ‘Activin A-Induced Differentiation of Embryonic Stem Cells into Endoderm and Pancreatic Progenitors—The Influence of Differentiation Factors and Culture Conditions’, Stem Cell Rev. Rep., vol. 5, no. 2, pp. 159–173, Jun. 2009, doi: 10.1007/s12015-009-9061-5.
[8] G. Hübner, Q. Hu, H. Smola, and S. Werner, ‘Strong Induction of Activin Expression after Injury Suggests an Important Role of Activin in Wound Repair’, Dev. Biol., vol. 173, no. 2, pp. 490–498, Feb. 1996, doi: 10.1006/dbio.1996.0042.
[9] H. A. Loomans and C. D. Andl, ‘Intertwining of Activin A and TGFβ Signaling: Dual Roles in Cancer Progression and Cancer Cell Invasion’, Cancers, vol. 7, no. 1, Art. no. 1, Mar. 2015, doi: 10.3390/cancers7010070.
[10] K. L. Jones, D. M. de Kretser, Shane. Patella, and D. J. Phillips, ‘Activin A and follistatin in systemic inflammation’, Mol. Cell. Endocrinol., vol. 225, no. 1, pp. 119–125, Oct. 2004, doi: 10.1016/j.mce.2004.07.010.
[11] X. Wang, G. Fischer, and M. Hyvönen, ‘Structure and activation of pro-activin A’, Nat. Commun., vol. 7, no. 1, Art. no. 1, Jul. 2016, doi: 10.1038/ncomms12052.
[12] L. S. Mathews, ‘Activin Receptors and Cellular Signaling by the Receptor Serine Kinase Family’, Endocr. Rev., vol. 15, no. 3, pp. 310–325, Jun. 1994, doi: 10.1210/edrv-15-3-310.
[13] A. E. Harrington, S. A. Morris-Triggs, B. T. Ruotolo, C. V. Robinson, S. Ohnuma, and M. Hyvönen, ‘Structural basis for the inhibition of activin signalling by follistatin’, EMBO J., vol. 25, no. 5, pp. 1035–1045, Mar. 2006, doi: 10.1038/sj.emboj.7601000.
[14] C. Welt, Y. Sidis, H. Keutmann, and A. Schneyer, ‘Activins, inhibins, and follistatins: from endocrinology to signaling. A paradigm for the new millennium’, Exp. Biol. Med. Maywood NJ, vol. 227, no. 9, pp. 724–752, Oct. 2002, doi: 10.1177/153537020222700905.
[15] K. A. D’Amour, A. D. Agulnick, S. Eliazer, O. G. Kelly, E. Kroon, and E. E. Baetge, ‘Efficient differentiation of human embryonic stem cells to definitive endoderm’, Nat. Biotechnol., vol. 23, no. 12, Art. no. 12, Dec. 2005, doi: 10.1038/nbt1163.
[16] L. Andreasson, H. Evenbratt, R. Mobini, and S. Simonsson, ‘Differentiation of induced pluripotent stem cells into definitive endoderm on Activin A-functionalized gradient surfaces’, J. Biotechnol., vol. 325, pp. 173–178, Jan. 2021, doi: 10.1016/j.jbiotec.2020.10.030.
[17] S. Ghorbani-Dalini et al., ‘Optimization of activin-A: a breakthrough in differentiation of human induced pluripotent stem cell into definitive endoderm’, 3 Biotech, vol. 10, no. 5, p. 215, Apr. 2020, doi: 10.1007/s13205-020-02215-3.
[18] S. Qiu et al., ‘An Efficient Method for the Differentiation of Human iPSC-Derived Endoderm toward Enterocytes and Hepatocytes’, Cells, vol. 10, no. 4, Art. no. 4, Apr. 2021, doi: 10.3390/cells10040812.
[19] C. Arber et al., ‘Activin A directs striatal projection neuron differentiation of human pluripotent stem cells’, Development, vol. 142, no. 7, pp. 1375–1386, Apr. 2015, doi: 10.1242/dev.117093.
[20] C. C. Miranda, T. G. Fernandes, M. M. Diogo, and J. M. S. Cabral, ‘Towards Multi-Organoid Systems for Drug Screening Applications’, Bioengineering, vol. 5, no. 3, Art. no. 3, Sep. 2018, doi: 10.3390/bioengineering5030049.
[21] L. Shariati, Y. Esmaeili, S. Haghjooy Javanmard, E. Bidram, and A. Amini, ‘Organoid Technology: Current Standing and Future Perspectives’, Stem Cells, vol. 39, no. 12, pp. 1625–1649, Dec. 2021, doi: 10.1002/stem.3379.
Publications
Distinct pathways drive anterior hypoblast specification in the implanting human embryo.
In Nature Cell Biology on 1 March 2024 by Weatherbee, B. A. T., Weberling, A., et al.
Self-renewing human naïve pluripotent stem cells dedifferentiate in 3D culture and form blastoids spontaneously.
In Nature Communications on 22 January 2024 by Guo, M., Wu, J., et al.
Pluripotent stem cell-derived model of the post-implantation human embryo.
In Nature on 1 October 2023 by Weatherbee, B. A. T., Gantner, C. W., et al.
Transgene directed induction of a stem cell-derived human embryo model.
Preprint on BioRxiv : the Preprint Server for Biology on 15 June 2023 by Weatherbee, B. A., Gantner, C. W., et al.
Differentiating functional human islet-like aggregates from pluripotent stem cells.
In STAR Protocols on 16 December 2022 by Barsby, T., Ibrahim, H., et al.
The HASTER lncRNA promoter is a cis-acting transcriptional stabilizer of HNF1A.
In Nature Cell Biology on 1 October 2022 by Beucher, A., Miguel-Escalada, I., et al.
Pancreas agenesis mutations disrupt a lead enhancer controlling a developmental enhancer cluster.
In Developmental Cell on 22 August 2022 by Miguel-Escalada, I., Maestro, M. A., et al.
Stem cell-derived porcine macrophages as a new platform for studying host-pathogen interactions.
In BMC Biology on 14 January 2022 by Meek, S., Watson, T., et al.
FAQ
Activin A is a multifunctional protein. It is involved in regulating embryonic development, cell proliferation, and differentiation. It promotes the patterning and differentiation of various organs, including the development of the mesoderm, neural, and reproductive systems. It is also involved in maintaining homeostasis, regulating immune responses, and wound healing. Finally, it stimulates the release of follicle-stimulating hormone from the pituitary gland.
Activin A is a critical factor in stem cell culture, commonly used to maintain the pluripotency of human induced pluripotent stem cells (iPSCs) and human embryonic stem cells (ESCs). It is integral to various stem cell differentiation protocols, guiding the differentiation of the definitive endoderm, neural and mesodermal lineages. Activin A is also commonly utilized in the development and maintenance of organoids.
Activin A and inhibin A are closely related proteins in the TGF-β superfamily. They share structural similarities but have distinct functions and roles in regulating various physiological processes. Activin A has broader functions beyond reproduction, which include handling embryonic development, stem cell maintenance, and the immune system. Inhibin A, conversely, is more specifically associated with the feedback control of follicle-stimulating hormones in the context of reproductive physiology.
Activin A binds to activin type I (ALK4 or ALK7) and type II (ActRIIA or ActRIIB) receptors to activate downstream SMAD signalling.
Yes, activin is involved in a feedback loop that regulates the secretion of follicle-stimulating hormone (FSH) and luteinising hormone (LH).
The activin gene family includes several genes that encode different activin subunits, forming various activin isoforms such as Inhibin Beta Subunits (INHBA / INHBB / INHBC / INHBD).
Follistatin and inhibins tightly regulate activin A activity in a feedback loop.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.
Share your thoughts!
Let us know what you think...
What others are saying



Qkine Activin A works nicely for human pluripotent stem cell differentiation to endoderm
Stem cell researcher, University of Oxford, UK –
We are using Qkine Activin A and it works nicely for human pluripotent stem cell differentiation to endoderm. University of Oxford, UK.
Upvote if this was helpful (0) Downvote if this was not helpful (0) Flag for removal