Currency
Recombinant human GDF-15 protein (Qk017)
Human growth differentiation factor 15 (GDF-15) protein is a member of the TGFβ family and subject of intense interest as a marker of cellular stress and for its role in metabolism, cancer and pregnancy. Human GDF15 also is functional in mouse studies.
Qkine GDF-15 is a 25 kDa disulfide-linked dimer composed of the mature domain of human GDF-15 protein. Our recombinant GDF15 protein is exceptionally high purity, animal origin-free and extensively validated to ensure no trace contamination of related TGFβ family proteins from the mammalian culture systems.
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
1000µg will be despatched as 2 x 500µg
Fast and free shipping.
Buy online with secure credit card or purchase order.
For any questions, please email orders@qkine.com
Summary
High purity human GDF15 protein (Uniprot: Q99988)
>98%, by SDS-PAGE quantitative densitometry
25 kDa (dimer). Mature active protein is a disulfide-linked dimer.
Expressed in E. coli
Animal origin-free
Carrier protein-free
Manufactured in our Cambridge, UK laboratories
Lyophilized from acetonitrile, TFA
Resuspend in 10mM HCl at >100 µg/ml (provided with protein and free of charge), prepare single use aliquots, add carrier protein if desired and store frozen at -20°C or -80°C
Featured applications
Biomarker for cellular stress
In vivo metabolic studies in mice (using human GDF15)
human
species similarity:
mouse – 67%
porcine – 67%
rat – 66%
bovine – 66%
Bioactivity
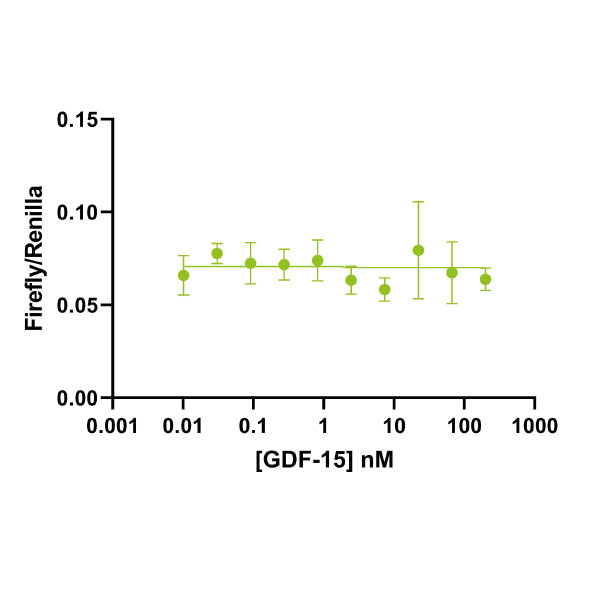
GDF15 signals through GRAL and co-receptor RET leading to RET phosphorylation and signalling through the ERK and AKT pathway (reviewed in Emmerson et al., 2018). Commercial sources of GDF15, in particular those purified from mammalian expression systems, have been shown previously to be contaminated with trace amounts of TGFβ. These trace contaminants cause misleading experimental results due to the picomolar or even femtomolar EC50s (Olsen et al., 2017). Here we use a well-characterized SMAD2/3 activation assay to show that there is no contamination from other TGFβ family proteins. Bioactivity is determined using a luciferase reporter assay in HEK293T cells. Cells are treated (in triplicate) with a serial dilution of GDF15 or Qk010 TGFβ1 for 6 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. EC50 = 0 pM (no contamination with TGFβ or related growth factors). Data from Qk017 lot #104282
Purity
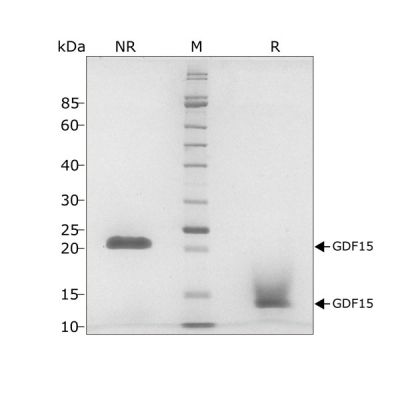
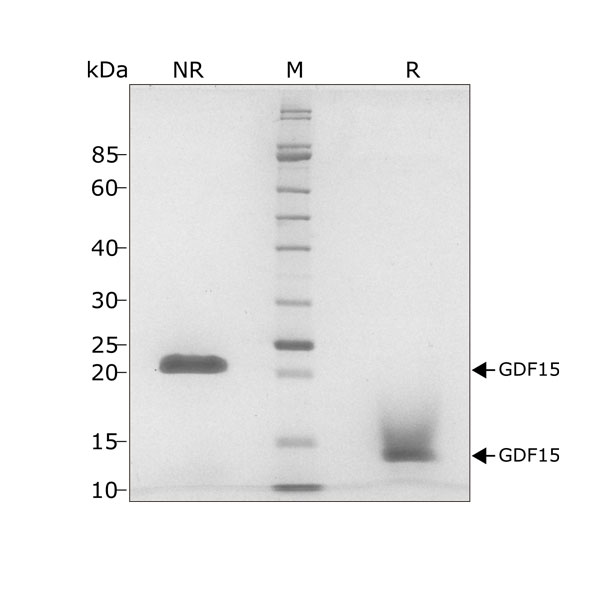
GDF15 migrates as a single band at 24 kDa in non-reducing (NR) and 13 kDa as a single monomeric species upon reduction (R). No contaminating protein bands are visible. Purified recombinant protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250. Data from Qk017 lot #010
Further quality assays
Mass spectrometry: single species with expected mass
Analytical reversed-phase: single sharp peak
Endotoxin: <0.005 EU/μg protein (below level of detection)
Recovery from stock vial: >95%
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
Growth differentiation factor 15 (GDF-15) is a distant member of the TGFβ superfamily. Its expression is tightly regulated and circulating GDF15 protein in serum is associated with diseases such as cancer, cardiovascular disease, obesity and metabolic disease. GDF15 protein is being recognized as an important biomarker for cellular stress.
Unlike other members of the TGFβ superfamily that cause activation of the SMAD pathway, GDF15 protein signals through GRAL and co-receptor RET leading to RET phosphorylation and signalling through the ERK and AKT pathway [1]. Commercial sources of recombinant human GDF15 protein, in particular those purified from mammalian expression, are frequently contaminated with trace amounts of TGFβ and related proteins. These trace contaminants cause misleading experimental results due to the picomolar or even femtomolar EC50s of this family of cytokines [2]. Please be cautious with your source of recombinant GDF15 protein, our scientists are happy to provide further information, please email support@qkine.com
We produce our proteins in E. coli with no animal products in our culture or purification processes to ensure there is no contamination from related proteins. In addition, we use a well-characterized SMAD2/3 activation assay to confirm there is no SMAD signalling.
Publications
GDF15 linked to maternal risk of nausea and vomiting during pregnancy
In Nature on 13 December 2023 by Fejzo, M., Rocha, N., Cimino, I. et al.
Fetally-encoded GDF15 and maternal GDF15 sensitivity are major determinants of nausea and vomiting in human pregnancy
Preprint 4 June 2023 by Fejzo, M. et al
The Common H202D Variant in GDF-15 Does Not Affect Its Bioactivity but Can Significantly Interfere with Measurement of Its Circulating Levels
In The Journal of Applied Laboratory Medicine November 2022 by Karusheva, Y. et al.
Activation of the hypothalamic–pituitary–adrenal axis by exogenous and endogenous GDF15
In PNAS on 29 June 2021 by Cimino, I. et al.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.



What others are saying
There are no contributions yet.