 Recombinant human FGF-10 protein (Qk003)
Recombinant human FGF-10 protein (Qk003)Recombinant human FGF-10 protein (Qk003)
£125.00 – £1,400.00

Human fibroblast growth factor 10 (FGF-10) protein promotes lung organoid formation and induces branching morphology. FGF-10 protein is used widely in organoid culture, embryonic stem cell (ESC) and induced-pluripotent stem cell (iPSC) differentiation, and for the study of epithelial to mesenchymal transition (EMT) and tumor metastasis.
Qkine Qk003 is a high purity and bioactivity 17 kDa bioactive domain of human FGF-10, animal origin-free (AOF) and carrier-protein free (CF).
In stock
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
£125.00 – £1,400.00
Fast and free shipping.
Buy online with secure credit card or purchase order. For any questions, please email orders@qkine.com
Summary:
- High purity human FGF-10 protein (residues 64-208, Uniprot: O15520)
- 17 kDa
>98%, by SDS-PAGE quantitative densitometry
Expressed in E. coli
Animal origin-free (AOF) and carrier protein-free
Manufactured in our Cambridge, UK laboratories
Lyophilized from HEPES, NaCl, mannitol
- Resuspend in sterile-filtered water at >50 µg/ml, add carrier protein if desired, prepare single use aliquots and store frozen at -20 °C (short-term) or -80 °C (long-term).
Featured applications:
Differentiation of embryonic stem cells into gut-like structures, cardiomyocytes and hepatocytes
Epithelial to mesenchymal transition
- Recombinant human EGF protein (Qk011)
- Recombinant human noggin protein (Qk034)
- Recombinant human R-spondin 1 protein (Qk006)
- Recombinant human HGF NK1 protein (Qk013)
- Recombinant mouse noggin protein (Qk033)
- Recombinant human KGF (FGF-7) protein (Qk046)
- Recombinant porcine EGF protein (Qk064)
- Recombinant human FGF-4 protein (Qk004)

FGF-10 activity was determined using the firefly luciferase reporter assay in stably transfected HEK293T cells. Cells are treated in triplicate with a serial dilution of FGF-10. Firefly luciferase activity is measured and normalized. EC50 = 0.36 ng/ml (21.1 pM). Data from Qk003 lot #104403.
FGF-10 migrates as a single band at 17 kDa in non-reducing (NR) conditions and upon reduction (R). No contaminating protein bands are visible. Purified recombinant human FGF-10 protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250. Data from Qk003 lot #010.

Further quality assays
Mass spectrometry: single species with expected mass
Recovery from stock vial: >95%
Endotoxin: <0.005 EU/μg protein (below level of detection)
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
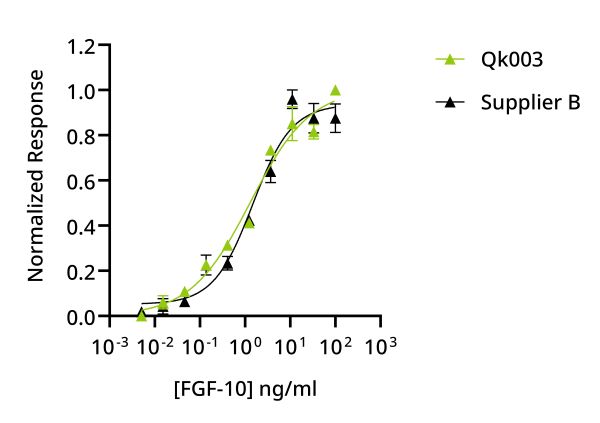
Qkine FGF-10 is as biologically active as a comparable alternative supplier protein. Quantitative luciferase assay with Qkine FGF-10 (Qk003, green) and alternative supplier FGF-10 (Supplier B, black). Cells were treated in triplicate with a serial dilution of FGF-10 for 4 hours. Firefly luciferase activity was measured and normalized to control Renilla luciferase activity.
Technote | FGF-10 (Qk003) bioactivityProtein background
The mature form of human FGF-10 protein is an approximately 20 kDa protein highly similar to FGF-7 and with a serine-rich region near its N-terminus [1]. It is secreted by mesenchymal cells and is bound and activated by extracellular FGF-BP [2]. Human FGF10 protein is expressed in the mesenchyme and functions through interacting with the epithelial FGF Receptor 2b (FGFR 2b) [3]. It has also been shown to interact weakly with FGFR 1b [4].
Human fibroblast growth factor 10 is first active in the limb bud mesoderm where it creates and maintains FGF signaling with epithelial FGF-8, then drives a positive feedback loop accumulating mesenchyme in the growing bud, and finally induces the apical ectodermal ridge which ultimately gives rise to feet and hands [5]. Lung development is based on the same epithelial-mesenchymal FGF mediations involving FGF-10 from the foregut mesenchyme signaling to FGFR2 in the foregut epithelium [6]. Furthermore, FGF-10 protein is involved in the development of white adipose tissue, heart, liver, brain, kidney, thymus, inner ear, tongue, trachea, eye, prostate, salivary gland and mammary gland. It has been shown to induce migration and invasion of pancreatic cancer cells and to be associated with breast cancer risk, and patients with FGF-10 haploinsufficiency present symptoms of chronic obstructive pulmonary disease [3].
FGF-10 is involved in a number of different embryo and adult cell and tissue types, including mesenchymal, neuronal and epithelial cells. Human recombinant FGF-10 protein drives the differentiation of embryonic stem cells (ESC) into gut-like structures, cardiomyocytes and hepatocytes [3]. It is also a potent factor in the development of organoids, increasing organoid size and branching phenotype compared to other FGFs [7].
Customer & collaborator data
FGF-10 supports epithelial to mesenchymal transition (EMT) in human primary keratinocytes. Data and evaluation by Stemnovate Ltd. EMT is a crucial morphogenetic process during development in which cells lose their epithelial characteristics and acquire migratory mesenchymal properties. Human FGF-10 protein has an important role both during the embryonic EMT (type I) and on cancer cell initiation of metastasis (type III EMT).


Induction of EMT in human primary keratinocytes following treatment with human FGF-10. Induction of EMT was evaluated using immunofluorescence staining to determine expression of the epithelial marker Cytokeratin 14 (CK14) and mesenchymal marker α-Smooth Muscle Actin (αSMA) in human primary epidermal keratinocytes after 4 days treatment with Qk003 human FGF-10 (0-100 ng/ml). FGF-10 supports proliferation and promotes epithelial to mesenchymal transition in human primary keratinocytes. Data and evaluation by Stemnovate Ltd.
Cell proliferation assays to assess the effect of Qkine FGF-10 (0-100 ng/ml) on human primary epidermal keratinocytes in serum-free keratinocyte media. Cells were evaluated at culture days: 0 (baseline), 1, 2, 3, 4 days, as summarized schematically in Figure 1a. Figure 1b shows cell proliferation for days 1, 2, 3, 4 and normalized to day 0 readouts (n=3; P*<0.05 vs control). The log concentration plot in Figure 1c shows percent cell proliferation normalized over untreated control (%) and to day 0 (baseline) after 4 days treatment (n=3; P*<0.05). The maximal cell proliferation was observed at ~10 ng/ml FGF-10 and a reduction in cell number/viability as observed at 100 ng/ml. Data provided by Stemnovate Ltd, Cambridge, UK.


Human FGF-10 allowed us to derive and maintain patient-derived gastric organoids as part of our cocktail. Thanks to the high purity of their cytokines and quality-price ratio overcome by far our usual providers. Data from Dylan Liabeuf.
Publications using Recombinant human FGF-10 protein (Qk003)
-
Generation of human iPSC-derived pancreatic organoids to study pancreas development and disease
Darrigrand J-F, Isaacson A and Spagnoli FM
DOI: https://doi.org/10.1101/2024.10.24.620102 -
Human epidermis organotypic cultures, a reproducible system recapitulating the epidermis in vitro
Agarwal R, Dittmar T, Beer HD et al.
DOI: doi: 10.1111/exd.14823 -
mTOR activity paces human blastocyst stage developmental progression
Iyer DP, Khoei HH, van der Weijden VA et al.
DOI: doi: 10.1016/j.cell.2024.08.048
FAQ
FGF-10 is essential for normal embryonic development, particularly in limb bud development. FGF family growth factors have roles in cell survival and proliferation, angiogenesis, tumorigenesis, wound healing and tissue repair.
FGF-10 is expressed in all branching morphogen organs such as the lungs, skin, ear and salivary glands.
FGF-10 stimulates cell survival and proliferation on many cell types including lung and gut epithelium but has no effect on fibroblasts.
FGF-10 binds to and signals though FGF receptors 2b and 1b.
FGFRs phosphorylate specific tyrosine residues and activate the RAS-MAPK, PI3K-AKT, PLCγ, and STAT intracellular signaling pathways.
FGF-10 drives the differentiation of embryonic stem cells into gut-like structures, cardiomyocytes and hepatocytes. It allows development of large organoids with branching phenotypes.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.

Receive an Amazon gift voucher when you leave us a review.
£25, $30 or €30 for reviews with an image and £10, $15 or €15 for reviews without an image