Recombinant human/mouse/rat/bovine/porcine BMP-2 protein (Qk007)
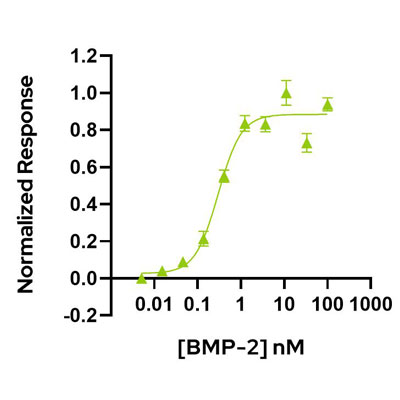
Human/mouse/rat/bovine/porcine BMP-2 protein (bone morphogenetic protein 2) protein is member of the TGFβ family and a key regulator of embryogenesis and potent differentiation factor of embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC) towards endoderm fates. BMP-2 plays roles in the differentiation of mesenchymal cells to adipocytes, epithelial cancer EMT, chondrogenesis and regulation of neuronal and glial cell development.
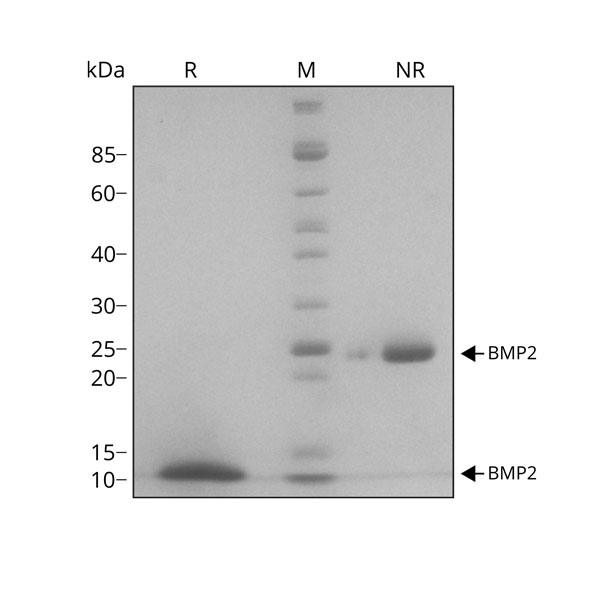
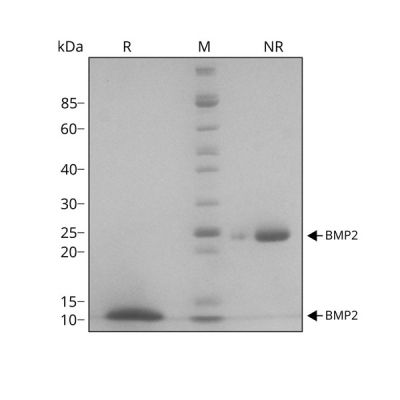
26 kDa disulfide–linked bioactive highly pure dimer comprised of the mature domain of human BMP-2 protein. Animal origin-free (AOF) and carrier protein-free.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.


What others are saying
There are no contributions yet.