FGF family
Fibroblast growth factors (FGFs) are a family of 22 proteins (18 secreted proteins and 4 intracellular FGFs) involved in many developmental processes. Fibroblast Growth Factors stimulate growth or differentiation of cells of mesodermal or neuroectodermal origin. Qkine FGF portfolio includes many members of the FGF superfamily: FGF-1, FGF-2, FGF-4, FGF-7, FGF-8a, FGF-8b, FGF-9, FGF-10 and FGF-18.
FGF-2
From the lab of Elena Carbognin and Graziano Martello, University of Padua
From the labs of Catherine H. Wilson, University of Cambridge and James E. Hudson, QIMR Berghofer Medical Research Institute
From the labs of Jamie A. Hackett, European Molecular Biology Laboratory EMBL-Rome, Davide Cacchiarelli, Telethon Institute of Genetics and Medicine and Graziano Martello, University of Padua.
From the lab of Elisabetta Ferretti, University of Copenhagen
From the lab of Graziano Martello, University of Padova
From the lab of Austin Smith, University of Cambridge & University of Exeter
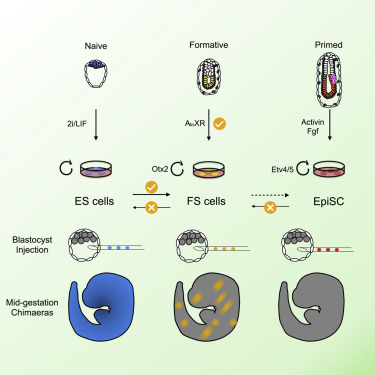
In the study of embryonic stem cells, stem cells representative of naïve and primed pluripotency have been well established in the forms of embryonic stem cells (ESCs) and epiblast-derived stem cells (EpiSCs). In this study Kinoshita et al. fill the gap between early and late pluripotency in describing an intermediate state; formative stem (FS) cells. FS cells differ from both ESCs and EpiSCs, a difference beautifully exemplified by their relative contribution to chimeras. Compared with ESCs, which readily contribute to chimeras, FS chimera contribution is less frequent, and their contribution is less evenly distributed. EpiSCs on the other hand do not generally contribute to chimeras at all. FS cells were established by culturing E5.5 epiblasts, or ES cells, in N2B27 media supplemented with a low dose of Qkine Activin A alongside a Wnt inhibitor and pan-retinoic acid receptor inverse agonist. We are proud our growth factors could be part of such an exciting finding!
Masaki Kinoshita, first author, MRC Cambridge Stem Cell Institute, University of Cambridge, says:
“Formative” pluripotency exists transiently in early development and naive mouse ES cell differentiation, which cells directly respond to differentiation signals. This paper showed that formative pluripotency is now captured in culture and expands its knowledge including chimaera competency of early embryonic cells.
Luo, L, et al. Hydrostatic Pressure Promotes Chondrogenic Differentiation and Microvesicle Release from Human Embryonic and Bone Marrow Stem Cells
Biotechnology Journal (2021).
From the lab of Alicia El Haj, University of Birmingham
From the lab of Tom Burdon, University of Edinburgh
From the lab of Dr Marta Shahbazi, MRC Laboratory of Molecular Biology, Cambridge
Reviewers comments available to view: Stadtfeld, M. Evaluation of Stuart et al.: Distinct Molecular Trajectories Converge to Induce Naive Pluripotency. Cell Stem Cell 25, 297–298 (2019). doi: 10.1016/j.stem.2019.08.009
From the lab of José Silva, University of Cambridge
From the lab of Daniel J. Fazakerley, University of Cambridge
From the lab of Ludovic Vallier, University of Cambridge
Used:
FGF-2 / bFGF 154 aa (Qk027)
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
From the lab of Anthony Davenport, University of Cambridge
Used:
zebrafish FGF-2 (Qk002)
DOI: doi: 10.1038/s42003-021-02453-y.
From the lab of Anthony Davenport, University of Cambridge
Used:
zFGF-2 / bFGF (Qk002)
From the lab of Graziano Martello, University of Padua
FGF2-G3
Cytotherapy (2024).
From the lab of Robert Thomas, Loughborough University
Robert Thomas’s lab at Loughborough University has analysed growth dynamics between commonly occurring genetically variant hPSCs and their counterpart wild-type cells in culture cells using proprietary computational modelling, allowing the identification of critical process parameters that drive critical quality attributes when genetically variant cells are present within the system This fascinating paper highlights how the system parameters controlling independent growth behaviour of wild-type and genetic variant populations are altered when both populations exist within a co-culture environment by introducing an ordinary differential equation (ODE) framework. Findings reveal that variant cells exhibit selective growth and competitive advantage, influencing the behaviour of wild-type cells, particularly at higher culture densities. This computational model offers opportunities for defining operational protocols and timely detection of emerging variants, crucial for product release and risk management. It is clear to see the importance as it demonstrates the utility of computational models in understanding complex biological systems and informing manufacturing practices in hPSC-based therapies.
bioRxiv preprint (2024)
From the lab of Margareta T Wilhelm, Karolinska Institute, Stockholm, Sweden
Used:
FGF2-G3 (Qk053)
Cell (2024)
From the lab of Aydan Bulut-Karsliogl, Department of Genome Regulation, Max Planck Institute for Molecular Genetics
From the lab of Athina E. Markaki, University of Cambridge
Used:
FGF2-G3 (Qk053)
From the lab of Ivana Barbaric, University of Sheffield
Used:
FGF2-G3 (Qk053)
From the lab of Ivana Barbaric, University of Sheffield
Used:
FGF2-G3 (Qk053)
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
From the lab of Margareta Wilhelm, Karolinska Institutet
Used:
FGF2-G3 (Qk053)
The drug discovery process is reliant on appropriate models for high-throughput screening, this can be difficult when complex, heterogeneous diseases are the targeted indication. This publication reports on a fascinating zebrafish model for the study of medulloblastoma, one of the most common malignant brain tumors in children. Introduction of medulloblastoma cells into zebrafish embryos leads to tumor growth in the hindbrain region and the homing of transplanted cells and the aggressiveness of tumor growth were enhanced by pre-culturing cells in a neural stem cell-like medium. This model was then used to successfully assess the effect of anti-cancer drugs on the viability of medulloblastoma cells in this zebrafish embryo model.
KGF (FGF-7)
bioRxiv preprint 2024
From the lab of Professor Francesca Spagnoli, King’s College London, UK
FGF-10
From the labs of Emmanuel Contassot and Alexander A. Navarini, University of Basel
bioRxiv preprint 2024
From the lab of Professor Francesca Spagnoli, King’s College London, UK
Cell (2024)
From the lab of Aydan Bulut-Karsliogl, Department of Genome Regulation, Max Planck Institute for Molecular Genetics