Cellular agriculture has the potential to transform food production and impact the health and well-being of future generations. Cultivated meat, fish, fat and dairy produced by culturing animal cells has immense promise but there are many challenges that must be overcome to bring these technologies to market and achieve cost parity.
The cell culture media used to grow these cells is an intense area of R&D. Bioactive recombinant proteins, especially growth factors and cytokines, are critical components in most cultivated meat cell culture media, with the exception of some chicken and fish media. Growth factor proteins are highly potent and provide a carefully orchestrated set of biochemical signals to control cell growth and fate.
Immediate priorities for cultivated meat and fat culture media optimization are:
Are species-specific growth factors essential to produce cultivated meat?
Many human growth factors will also stimulate animal cells, although limited data are available to help understand the relative efficacy and specificity of cross-species bioactivity. However, recently there has been a strong recommendation that cultivated meat companies use growth factors from the same species, or as a minimum use a non-human growth factor. Although, as far as we are aware, there is no formal guidance available to date, this seems to be the clear direction of travel based on feedback from submissions by industry leaders and new verbal recommendations from the FDA (USA Food and Drug Administration). The use of species-matched bioactive proteins will undoubtedly smooth regulators’ consideration, aid consumer acceptance and likely also improve efficacy, therefore helping reduce the cost of media.
There is a pressing need for properly controlled comparative data demonstrating the relative efficacy of key growth factors on animal cells. To date, this has been a challenge due primarily to the lack of availability of commercial reagents, the variable quality of those reagents that are available and the lack of open-access comparative studies using recombinant proteins of equivalent biochemical quality and well-characterized cell lines.
As a first step to addressing the sector’s scientific and regulatory demands, we are developing and characterizing growth factors of identical biochemical quality from several relevant species. To this end we have developed a high quality food grade range of species-matched growth factors manufactured under a HACCP regime with full documentation such as allergen and beta-lactam statements, full raw material traceability, CoO and CoA for regulatory dossier preparation. In collaboration with other stakeholders, we hope to accelerate culture media development and generate open-access data for porcine and bovine iPSC, ESC and MSC culture.
Do we need new species-specific forms of all the growth factors used in bovine and porcine cell culture media?
Growth factors used commonly in cultivated bovine and porcine cell culture media were analyzed to identify proteins identical between the three species and those where the protein sequence isn’t conserved, which raises concerns over potential species-specific activity and regulatory needs.
This analysis included sequence conservation (Table 1) and functional domain analysis (data not shown). These data, along with industry priorities, have been used to prioritize the development of species-matched growth factors at Qkine. The following growth factors are used extensively in cultivated meat and fat culture media formulations and are not well-conserved between species. Here, we focus on FGF-2, TGF-β3 and HGF.
| Protein | Human product code | Human to porcine | Human to bovine | Food grade product code |
|---|---|---|---|---|
| Activin A | Qk001 | 100% | 100% | Qk108-FG |
| TGF-β1 | Qk010 | 100% | 100% | Qk111-FG |
| TGF-β2 | Qk072 | 100% | 100% | Qk112-FG |
| IGF-1 | Qk047 | 100% | 100% | Qk113-FG |
| NRG-1 | Qk045 | 100% | 100% | Qk115-FG |
Table 1a. Growth factors 100% conserved between human, bovine and porcine.
| Protein | Human product code | Human to porcine | Human to bovine | Species matched product(s) | Food grade product code(s) |
|---|---|---|---|---|---|
| EGF | Qk011 | ~85% | – | Porcine EGF Qk064 | Qk064-FG |
| FGF-2 (145 aa) | Qk025 | ~99% | ~99% | Bovine/porcine FGF-2 (145 aa) Qk040 | Qk040-FG |
| FGF-2 (154 aa) | Qk027 | ~99% | ~99% | Bovine/porcine FGF-2 (154 aa) Qk056 | Qk056-FG |
| HGF NK1 | Qk013 | ~95% | ~93% | Porcine HGF NK1 Qk061 Bovine HGF NK1 Qk060 | Porcine Qk061-FG Bovine Qk060-FG |
| LIF | Qk036 | 87% | 88% | In development | – |
| PDGF-BB | Qk044 | ~95% | ~91% | Bovine PDGF-BB Qk079 | Qk079-FG |
| TGF-β3 | Qk054 | ~98% | ~99% | Porcine TGF-β3 Qk084 | Qk084-FG |
Table 1b. Growth factor proteins not fully conserved between human, bovine and porcine.
Species-specific FGF-2 (bFGF) supplementation improves maintenance of pluripotency in porcine iPSCs and reveals differences in the efficacy of the two lengths of FGF-2
FGF-2 is commonly used to regulate cell proliferation and differentiation in induced pluripotent stem cell (iPSC) culture. FGF-2 is incorporated in the growth medium of ESCs and iPSCs to maintain cell pluripotency as either a short form (145 aa) or a longer form (154 aa), which comprises the core structured region and an N-terminal extension. Human FGF-2 is often used in animal stem cell growth media regardless of the cell species of origin. Perhaps unusually, the bioactivity of FGF-2 is highly functionally conserved between species (data not shown). The amino-acid sequence of bovine and porcine FGF-2 is identical and differs from the human protein by two amino acids.
Porcine iPSCs (piPSCs) can self-renew and differentiate into fat and muscle cells. These characteristics make them ideal candidates for cultivated meat applications. Developing an optimal growth media for piPSCs is essential in maintaining pluripotency during long-term cell culture. piPSCs require FGF-2 supplementation in their growth medium to maintain stemness.
In this study, we observed that both porcine and human Qkine FGF-2 variants demonstrate bioactivity conducive to the successful culture of piPSCs. Culturing piPSCs with either human or porcine FGF-2 isoforms resulted in the maintenance of normal colony morphology, as depicted in (Figure 1A). Interestingly, supplementation with both human and porcine 145 aa FGF-2 variants led to enhanced growth rates of piPSCs compared to those cultured in control piPSC medium (Figure 1B). However, the growth rates of porcine iPSCs were notably slower when exposed to standard maintenance medium supplemented with human or porcine 154 aa longer FGF-2 isoforms. Despite these variations, the expression of pluripotency markers OCT4 and SOX2 remained stable across all piPSC cultures supplemented with Qkine FGF-2 growth factors (Figure 1C). Notably, the 154 aa porcine FGF-2 variant (Qk056) exhibited the highest OCT4 to SOX2 ratio, supporting the potential benefits of utilizing porcine-specific growth factors in piPSC culture medium. Please note that control piPSC culture media already contains some human FGF-2 and further work is required to fully elucidate the mechanisms of the observed change in cell proliferation.
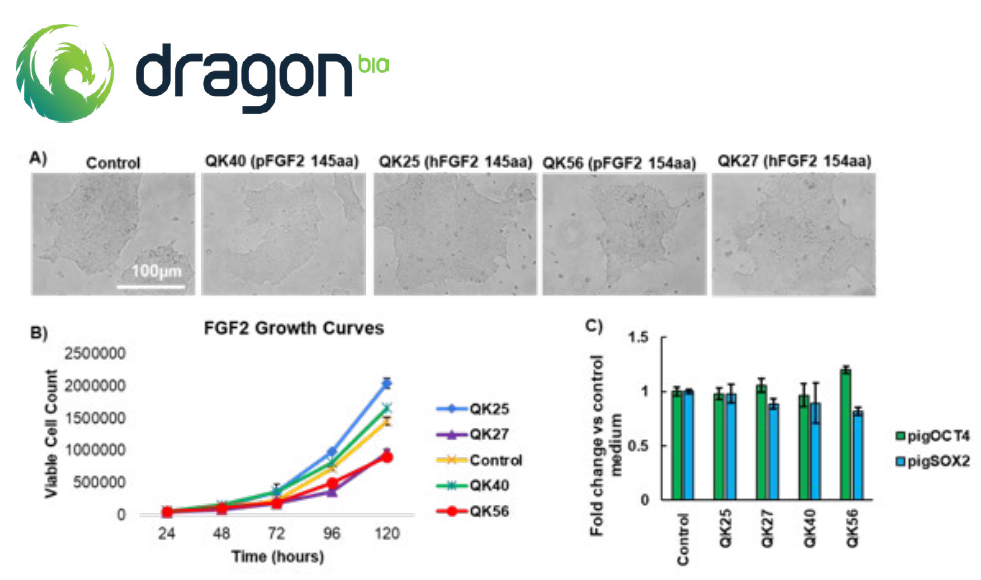
Figure 1: Comparison of porcine iPSCs cultured with human or bovine/porcine FGF-2. piPSCs were cultured on Geltrex coated 6-well plates for a minimum of 5 passages in porcine iPSC medium supplemented with FGF-2 at 100 ng/ml (Qk025, Qk027, Qk040 and Qk056). At passage 5 cells were imaged (A), then dissociated into single cells using Accutase and seeded at a density of 10,000 cells per well of a Geltrex coated 24 well plate in 500 μl of culture medium containing 1x revita cell supplement. The cells were fed daily over 5 days with 500 μl of culture medium containing each FGF-2 variant and triplicate cell counts performed daily over 5 days to establish growth curves (B). piPSCs were also harvested after the 5 passages and RNA collected for qPCR. mRNA was quantified using primers for porcine GAPDH (housekeeping), porcine OCT4 and SOX2 (pluripotency, C).
Thermostable bovine/porcine FGF-2 retains bioactivity after 7 days in culture allowing reduced frequency of media changes and improving compatibility with process scaleup
FGF-2 is inherently unstable and prone to proteolytic degradation and aggregation. This fundamental biochemical instability, and therefore low functional half-life in culture media (<10 h), is an important contributor to the need for frequent media changes and challenges in improving homogeneity during stem cell proliferation. The quality of the pluripotent stem cell culture has an important impact on the subsequent cell yield from differentiation and maturation processes.
Recombinant bovine/porcine FGF2-G3 protein is an engineered thermostable form of FGF-2 that supports the development of optimized species-specific serum free culture media. The use of human thermostable FGF2-G3 for improved iPSC feeding schedules is well documented, and the utility of this protein for improved scale-up and impact on homogeneity and controllability of cell mass production during bioprocessing scaleup is under investigation by the cellular agriculture community. However, protein tag-free forms of bovine/porcine FGF2-G3 compatible with manufacturing scaleup have not previously been available. In this study, the bioactivity of bovine thermostable FGF-2 was shown to be equivalent to wild-type bovine/porcine FGF-2 in a quantitative reporter assay (Figure 2). To assess the extension of the functional half-life of bovine/porcine FGF2-G3, the protein was incubated in conditioned media for 48 h to mimic cell culture conditions prior to bioactivity analysis. Thermostable bovine/porcine FGF2-G3 retains full activity after preincubation with conditioned media at 37°C for 48 h in contrast to wildtype protein activity, which degrades in culture (Figure 3).

Figure 2: Bovine/porcine wild-type (WT) and thermostable FGF-2 (FGF2-G3) have equivalent bioactivity. Bioactivity was determined in HEK293T cells using a serum response element luciferase reporter assay. Cells were treated in triplicate for 3 h with bovine/porcine FGF-2 (Qk040, black) and bovine/porcine FGF2-G3 (Qk080, green). WT FGF-2 (Qk040) had an EC50 of 0.237 ng/ml, FGF2-G3 (Qk080) had an EC50 of 0.190 ng/ml.
Our results demonstrate that FGF2-G3 has an increased functional half-life from <10 h (wild-type) to >48 h, similar to that observed with the human thermostable FGF2-G3 protein suggesting bovine/porcine FGF2-G3 is an appropriate alternative to human thermostable FGF-2 for cultivated meat media optimization. Please note, although engineered forms of proteins like FGF2-G3 offer unique promise for bioprocess optimization and scale-up, the use of engineered proteins may present additional regulatory hurdles. However, the transformative impact on cell yield, feeding schedules and homogeneity of cell cultures may warrant this additional dialogue with the regulators.
A

B
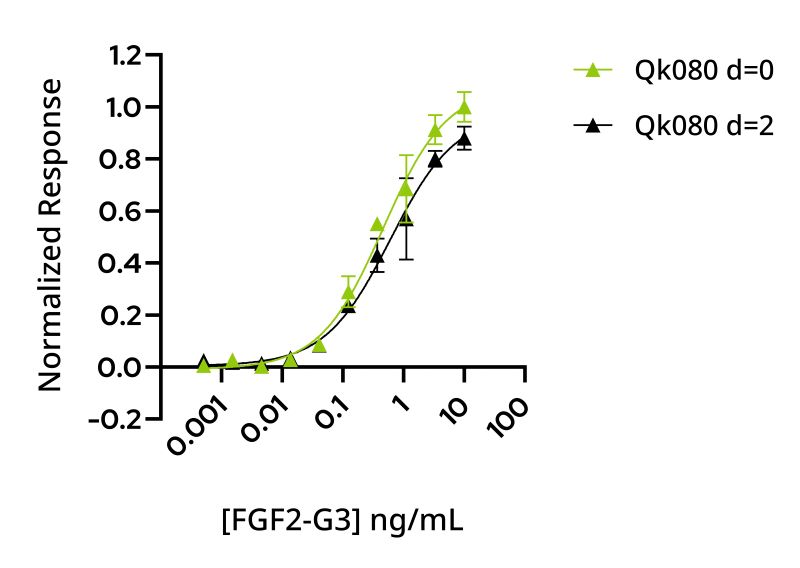
Figure 3: WT FGF-2 bioactivity is reduced after 2 days whereas thermostable FGF-2 maintains bioactivity. FGF-2 was diluted in conditioned media and incubated at 37 °C for 0 and 48 h before bioactivity determined by incubation for 3 h using a serum response element luciferase reporter assay in transfected HEK293T cells. (A) WT bovine/porcine FGF-2 (Qk040) at 0 h (green, EC50 0.237 ng/ml) and 48 h (black, EC50 1.75 ng/ml). (B) Bovine/porcine FGF2-G3 (Qk080) at 0 h (green, EC50 0.464 ng/ml) and 48 h (black, EC50 0.606 ng/ml). Results were normalized to the maximum response for 0 h.
TGF-beta family proteins and the impact of species-specific growth factors on porcine iPSC culture
The TGF-beta superfamily includes activin A, TGF-β1-3, GDFs, GDNF, and BMPs. This protein family plays a crucial role in governing many developmental and physiological processes. Activin A and TGF beta family proteins are often essential in cell culture media used in bovine and porcine cell culture. There are three closely related TGF-β proteins, TGF-β1, TGF-β2 and TGF-β3 that have distinct physiological roles, tissue distribution and developmental expression profiles in vivo. Distinct genes encode TGF-β 1–3 proteins, which are expressed in unique, occasionally overlapping, patterns and serve different functions in vivo. TGF-β proteins are potent signaling molecules that act primarily through the Smad 2/3-mediated pathway and perform crucial roles in cell proliferation, growth, differentiation and motility. TGF-β1 is 100% conserved in human, porcine and bovine, and used in many commercial ESC and iPSC media including Essential 8 and mTesR that form the basis of many culture media used in cultivated meat development. In contrast, TGF-β3 is not conserved between species (Figure 4) but is also used extensively in serum free media including B8 and the bovine variant, Beefy 9, and many mesenchymal and adult-derived animal stem cell media.
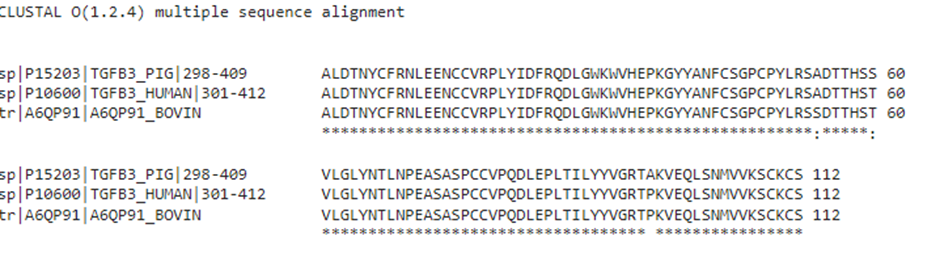
Figure 4: Multiple sequence alignment of porcine, bovine and human TGF-β3 demonstrate a ~98% sequence conservation between human and porcine TGF-β3, with a ~99% conservation between human and bovine TGF-β3.
It is important to note that this family of proteins are inherently difficult to manufacture due to their structural complexity. The active form of the protein is a disulfide-bonded dimer, and monomeric forms of the protein are not bioactive. The need for this family of growth factors is likely to eventually dominate the cost of goods related to bioactive recombinant protein media components as companies move to >50 l scale and then pilot phase.
Several innovative approaches are being explored to address this challenge. These include genetic approaches to remove or reduce the requirement for these proteins; co-culture and utilizing the secretion of these proteins by mammalian cells during culture, and developing optimized proteins and manufacturing processes to address the supply chain capacity and costs. The first step to improving the supply chain is developing reliable species-specific proteins and building on these data to develop engineered forms with improved manufacturing yield or biological properties. There is also potential to innovate in the protein manufacture process to tailor it specifically to the needs and scale of the cultivated meat industry. Porcine TGF-β3 was manufactured using an animal origin-free microbial fermentation process. The biological activity of this protein was compared directly with human TGF-β3 in a quantitative luciferase reporter assay on a human cell-line and showed equivalent bioactivity (Figure 5). This is promising and suggests a like-for-like substitution of human TGF-β3 with the porcine protein may be possible.
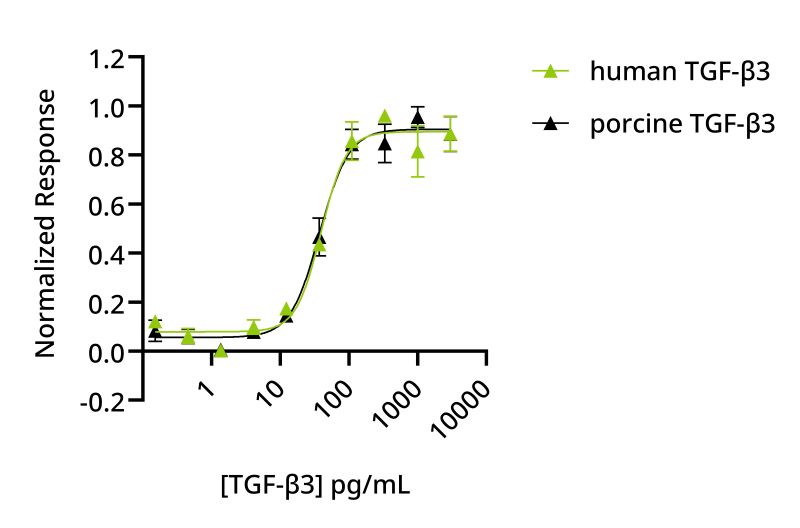
Figure 5: Human and porcine TGF-β3 have equivalent bioactivity. HEK293T cells transfected with a TGF-β3 responsive firefly luciferase reporter were treated in triplicate with a serial dilution of human TGF-β3 (Qk054, green) or porcine TGF-β3 (Qk084, black) for 6 h. Human TGF-β3 EC50 39.53 pg/ml, porcine TGF-β3 EC50 37.51 pg/ml.
Qkine TGF-β1 maintains pluripotency in piPSCs but pluripotency markers decrease in piPSC with human TGF-β3
As previously stated TGF-β1 has 100% homology across human, porcine and bovine, whereas TGF-β3 varies between species. piPSCs when exposed to either human/bovine/porcine TGF-β1 or human TGF-β3 during culture, demonstrated typical colony morphology (Figure 6A) and enhanced growth rates compared to piPSCs cultured under standard conditions with standard medium (Figure 6B). The expression levels of pluripotency markers OCT4 and SOX2 remain stable following TGF-β1 supplementation (Figure 6C).
However, importantly, the introduction of human TGF-β3 lead to a decrease in OCT4 expression, suggesting that human TGF-β3 in piPSCs may not adequately sustain pluripotency or may induce differentiation. Equivalent bioactivity of human and porcine proteins (Figure 5) suggests that it may be possible to directly substitute porcine TGF-β3 in media that currently contain human TGF-β3 to develop a species-matched culture media formulation. Further work using porcine TGF-β3 is required to determine the relative efficacy in porcine cell culture, which will be facilitated by the commercial availability of this growth factor.

Figure 6: Comparison of porcine iPSCs cultured with human/bovine/porcine TGF-β1 or human TGF-β3. piPSCs were cultured on Geltrex coated 6-well plates for a minimum of 5 passages in porcine iPSC medium supplemented with 2 ng/ml Qkine TGF-β1 (Qk010) or TGF-β3 (Qk054). At passage 5 cells were imaged (A), then dissociated into single cells using Accutase and seeded at a density of 10,000 cells per well of a Geltrex coated 24 well plate in 500 μl of culture medium containing 1x revita cell supplement. The cells were fed daily over 5 days with 500 μl of culture medium containing TGF-β1 or TGF-β3 and triplicate cell counts performed daily over 5 days to establish growth curves (B). piPSCs were also harvested after the 5 passages and RNA collected for qPCR. mRNA was quantified using primers for porcine GAPDH (housekeeping), porcine OCT4 and SOX2 (pluripotency, C).
HGF exhibits species-specific activity, suggesting published data using human HGF on bovine cells may warrant reevaluation
HGF is an important growth factor in bovine myoblast expansion and maturation media. Previously, Qkine developed an optimized animal-free native sequence active isoform of human HGF, HGF NK1, as all existing commercial HGF proteins were produced using animal or human cell protein expression systems, limiting their application in translational studies. To extend species-specific HGF availability, bovine and porcine HGF NK1 have been produced. The activity of human, bovine and porcine HGF NK1 proteins was compared using a reporter assay on human cells. Bovine HGF NK1 had reduced bioactivity compared to human HGF NK1 in human cells (Figure 7), suggesting potential species differences in HGF NK1 activity.
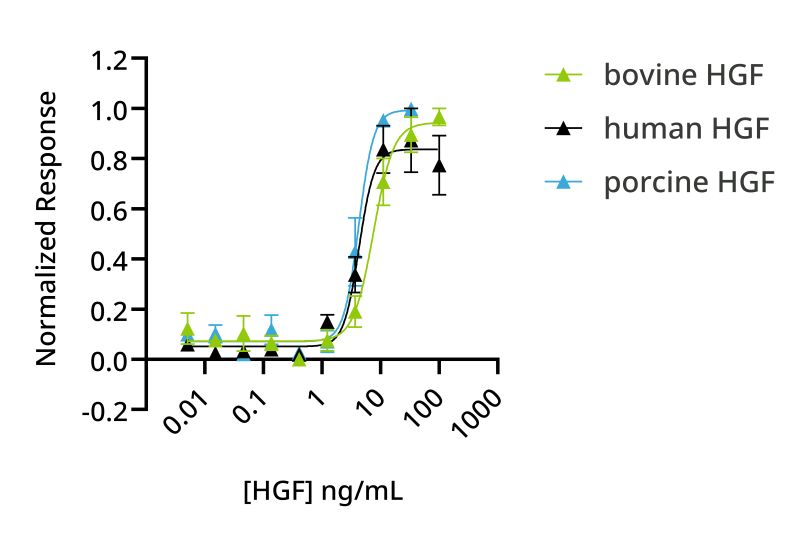
Figure 7: Comparison of human, porcine or bovine HGF NK1 bioactivity on human cells. Bioactivity was determined using a serum response element luciferase reporter assay in HEK293T cells. Cells were treated in triplicate with a serial dilution of human HGF NK1 (Qk013, black), porcine HGF NK1 (Qk061, blue) or bovine HGF NK1 (Qk060, green) for 3 hours. Firefly luciferase activity was measured and normalized to the control Renilla luciferase activity. EC50 human HGF NK1 = 1.56 ng/ml; EC50 porcine HGF NK1 = 3.86 ng/ml and EC50 bovine HGF NK1 = 7.71 ng/ml.
What is the future of growth factors for cultivated meat?
Cultivated meat, fish, fat and dairy are rapidly developing fields with the potential to impact global sustainability. Qkine is committed to supporting the cellular agriculture sector by extending the range of commercially available species-specific growth factors and supporting development of open collaborations, a scientific research base and credible long-term supply chain. Qkine species-specific high quality food grade growth factors have been developed to aid in regulatory dossier preparation, with full traceability of raw materials and extensive documentation. As with all Qkine growth factors these are subject to extensive QC and lot-to-lot bioactivity testing, conforming to our Nine Point Quality Commitment.
Visit qkine.com/cellular-agriculture for information on our high quality food grade range of proteins for cellular agriculture.
Further Information
Please contact our team: customerservice@qkine.com if you would like to discuss commercial or academic collaborations, supply agreements or any aspects of growth factor optimization for cultivated meat, fish, fat, dairy and other products.

