Activin proteins
Activins are part of the TGF–β superfamily of growth factors regulating embryonic development, cell proliferation, differentiation, endocrine function and immune responses. Qkine activin range includes activin A, used to maintain pluripotency in induced pluripotent and embryonic stem cell cultures and activin E, which is involved in hepatocyte proliferation and adipocyte function. Qkine have also engineered an optimised biologically active truncation of the mature domain of human activin A protein, activin A PLUS for cost-effective, large-scale stem cell culture applications.
Activin A
From the lab of Stina Simonsson, University of Gothenburg
Used:
activin A (Qk001)
In embryonic development, growth factors are delivered in a highly controlled and targeted manner, however when differentiating iPSCs the real challenge is to effectively mimic these conditions. Consequently, iPSC differentiation is plagued by issues such as low efficiency and a lack of homogeneity. In their recent paper Andreasson et al. take a step towards improving the differentiation of iPSCs to definitive endoderm. The group employs gold nanoparticles to generate a gradient of immobilised Activin A – a member of the TGF-β superfamily that plays a key role in definitive endoderm development. Using this gradient, the group was able to deliver Activin A in a controlled and localised manor, resulting in more efficient differentiation. By deploying their innovative approach, the group observed a dose dependent response of the cells to Activin A, as defined by expression of differentiation markers SOX17 and GATA4. Their results indicate that it may be possible to define an optimal density of Activin A for definitive endoderm differentiation – a finding that could improve the homogeneity and speed of differentiation. This innovative study is a wonderful example of how reconsidering the way in which growth factors are delivered can lead to advances in our understanding of the precise control of stem cell differentiation and how these cells undertake their fate decisions.
bioRxiv preprint (2024)
From the lab of Dr Jennifer Nichols, University of Cambridge
From the lab of Magdalena Zernicka-Goetz, University of Cambridge
Used:
activin A (Qk001)
From the lab of Timo Otonkoski, University of Helsinki
Used:
activin A (Qk001)
From the lab of Jorge Ferrer, Centre for Genomic Regulation (CRG)
Used:
activin A (Qk001)
From the labs of Tamir Rashid, Imperial College London and Eileen Gentleman, King’s College London.
Used:
activin A (Qk001)
DOI: 10.1002/sctm.18-0084
From the lab of Tamir Rashid, Kings College London
Used:
activin A (Qk001)
From the labs of Catherine H. Wilson, University of Cambridge and James E. Hudson, QIMR Berghofer Medical Research Institute
From the labs of Jamie A. Hackett, European Molecular Biology Laboratory EMBL-Rome, Davide Cacchiarelli, Telethon Institute of Genetics and Medicine and Graziano Martello, University of Padua.
bioRxiv preprint 2024.
From the lab of Professor Francesca Spagnoli, King’s College London, UK
From the lab of Elisabetta Ferretti, University of Copenhagen
From the lab of José Silva, Guangzhou Laboratory

A huge challenge in understanding human early embryo cell fate is due to limited access and ethical concerns. Recent research, however, from José C. R. Silva’s lab at Guangzhou National Laboratory, drawing from single-cell sequencing, suggests a conserved lineage specification process between human and mouse embryos. Blastoids, emerging models for early embryo development, generated solely from hnPSCs, offer insights into blastocyst formation without altering culture conditions. Self-renewing human naïve pluripotent stem cells (hnPSCs) spontaneously form blastoids in 3D culture, mimicking early human blastocysts. This process, mediated by the GSK3 inhibitor IM-12 in 5iLAF medium, involves upregulation of oxidative phosphorylation genes. hnPSCs dedifferentiate into E5 embryo-like intermediates, expressing SOX2/OCT4 and GATA6, which specify trophoblast fate by day 3, coinciding with blastoid formation. This was a fantastic paper to read as it is clear how this spontaneous blastoid formation highlights the importance of culture conditions and provides a new platform to study human embryo development in vitro, potentially reshaping our understanding of hnPSCs and embryo development.
From the lab of Kirmo Wartiovaara, University of Helsinki
Used:
activin A (Qk001)
From the lab of Santiago Vernia, LMS London Institute of Medical Sciences
Used:
activin A (Qk001)
Luo, L, et al. Hydrostatic Pressure Promotes Chondrogenic Differentiation and Microvesicle Release from Human Embryonic and Bone Marrow Stem Cells
Biotechnology Journal (2021).
From the lab of Alicia El Haj, University of Birmingham
From the lab of Jorge Ferrer, Centre for Genomic Regulation (CRG)
Used:
activin A (Qk001)
From the lab of Tom Burdon, University of Edinburgh
From the lab of Siim Pauklin, University of Oxford
Used:
activin A (Qk001)
From the lab of Cristina Pina, Brunel University
Used:
activin A (Qk001)
From the lab of Dr Marta Shahbazi, MRC Laboratory of Molecular Biology, Cambridge
Reviewers comments available to view: Stadtfeld, M. Evaluation of Stuart et al.: Distinct Molecular Trajectories Converge to Induce Naive Pluripotency. Cell Stem Cell 25, 297–298 (2019). doi: 10.1016/j.stem.2019.08.009
From the lab of José Silva, University of Cambridge
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
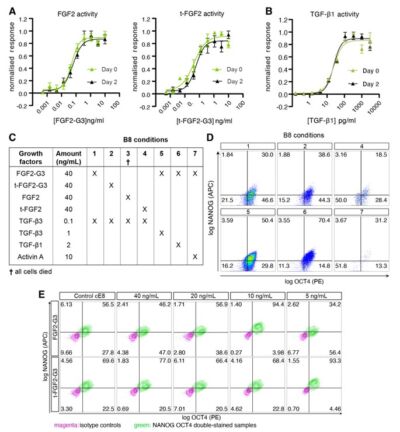
Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
From the lab of Harry Leitch, Imperial College London
Used:
activin A (Qk001)
From the lab of Magdalena Zernicka-Goetz, University of Cambridge
Used:
activin A (Qk001)
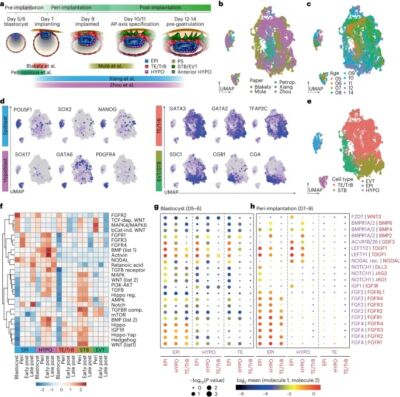
It has been a pleasure to read about the intricate dance of signaling coordination between the epiblast, trophectoderm, and hypoblast during early human embryonic development in this recent publication from the lab of Magdalena Zernicka-Goetz at the California Institute of Technology/University of Cambridge. Using human embryos and stem cell models, the research reveals NODAL dependency in anterior hypoblast specification, contrasting roles of BMP in mouse and human anterior signaling center maintenance, and the importance of NOTCH signaling in human epiblast survival. Comparative analysis highlights conserved and species-specific factors driving embryonic development. Specifically, NODAL, BMP, and NOTCH play crucial roles in anterior hypoblast formation, with signaling dynamics changing significantly post-implantation. The fantastic research underscores the complexity of signaling pathways during implantation and emphasizes the importance of further investigations to elucidate their roles comprehensively. These findings clearly contribute to understanding early human embryonic development and provide insights for improving stem cell-derived embryo-like models.
From the lab of Magdalena Zernicka-Goetz, University of Cambridge
Used:
activin A (Qk001)
From the lab of Graziano Martello, University of Padua
Activin A PLUS
From the lab of Austin Smith, University of Cambridge & University of Exeter
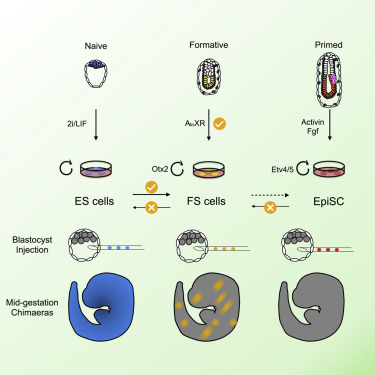
In the study of embryonic stem cells, stem cells representative of naïve and primed pluripotency have been well established in the forms of embryonic stem cells (ESCs) and epiblast-derived stem cells (EpiSCs). In this study Kinoshita et al. fill the gap between early and late pluripotency in describing an intermediate state; formative stem (FS) cells. FS cells differ from both ESCs and EpiSCs, a difference beautifully exemplified by their relative contribution to chimeras. Compared with ESCs, which readily contribute to chimeras, FS chimera contribution is less frequent, and their contribution is less evenly distributed. EpiSCs on the other hand do not generally contribute to chimeras at all. FS cells were established by culturing E5.5 epiblasts, or ES cells, in N2B27 media supplemented with a low dose of Qkine Activin A alongside a Wnt inhibitor and pan-retinoic acid receptor inverse agonist. We are proud our growth factors could be part of such an exciting finding!
Masaki Kinoshita, first author, MRC Cambridge Stem Cell Institute, University of Cambridge, says:
“Formative” pluripotency exists transiently in early development and naive mouse ES cell differentiation, which cells directly respond to differentiation signals. This paper showed that formative pluripotency is now captured in culture and expands its knowledge including chimaera competency of early embryonic cells.