BMPs and BMP antagonists
Bone morphogenic proteins (BMPs) are members TGFβ family and are important regulators of tissue development and growth. Qkine BMP range includes BMP-2 and BMP-4 which are key regulators of embryogenesis and potent differentiation factors for embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC).
BMP antagonists are important regulators of BMP activity in development, characterized by cysteine-rich domains and are divided into five types, the DAN family (gremlin, sclerostin, USAG-1, dante, cerberus and Coco), noggin, chordin, follistatin and twisted gastrulation. Qkine BMP antagonist range includes mouse/rat and human noggin and gremlin 1.
BMP-2
Huang, T et al. Sex-Specific Chromatin Remodelling Safeguards Transcription in Germ Cells
Nature, 600, 737–742 (2021)
From the lab of Petra Hajkova, MRC London Institute of Medical Sciences
Used:
BMP-2 (Qk007)
Luo, L, et al. Hydrostatic Pressure Promotes Chondrogenic Differentiation and Microvesicle Release from Human Embryonic and Bone Marrow Stem Cells
Biotechnology Journal (2021).
From the lab of Alicia El Haj, University of Birmingham
BMP-4
Azami, T. et al. STAT3 signalling enhances tissue expansion during postimplantation mouse development
bioRxiv preprint (2024)
From the lab of Dr Jennifer Nichols, University of Cambridge
From the lab of Elisabetta Ferretti, University of Copenhagen
From the lab of Daniel J. Fazakerley, University of Cambridge
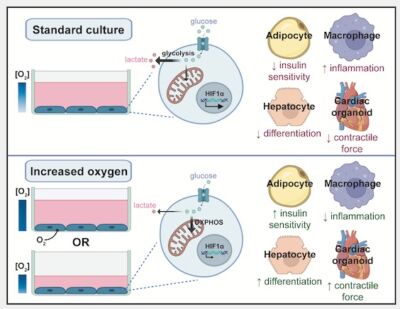
We all know our stem cell cultures are sensitive and high maintenance, we feed them, keep them warm to make sure we get the most accurate, reproducible data. The composition of their media is important, we need the right growth factors, but this week’s Friday read highlights that their exposure to oxygen can be an important factor in the differentiation and function of cellular models. In this very interesting read from the MRC Institute of Metabolic Science they have investigated the impact of oxygen diffusion and media volume on hypoxia-related transcriptional changes in stem cell cultures. They found that decreasing the media volume decreased lactate production and HIF1α expression and increased the functionality of adipocyte, hiPSC-derived hepatocytes and hiPSC-derived cardiac organoid cultures.
From the lab of Alessandro Bertero, University of Turin in collaboration with Qkine
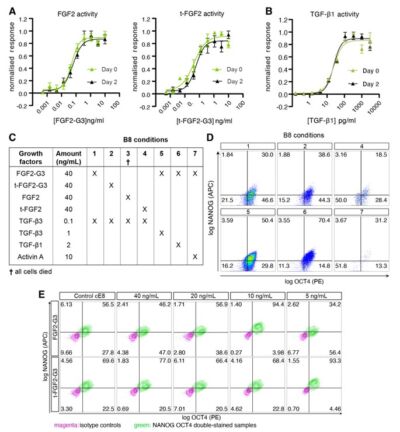
Cell therapy is becoming a possibility for many previously untreatable conditions, and it should be accessible to everyone. Creating a cost-effective, reliable and reproducible way of culturing human induced pluripotent stem cells (hiPSCs) in a range of research labs, and allowing large scale culture for gene-editing purposes takes us one step closer to this.
Using high potency thermostable Qkine 145 amino acid FGF-G3 reduce FGF-2 use 8-fold and for weekend-free culture reduced media use by 57%. This makes hiPSCs a more accessible model for many labs doing basic and translational research.
Gremlin 1
From the lab of Marta Shahbazi, MRC Laboratory of Molecular Biology, Cambridge
Used:
gremlin 1 (Qk015)
Noggin
From the labs of Emmanuel Contassot and Alexander A. Navarini, University of Basel
From the lab of Thorsten E. Boroviak, University of Cambridge
Used:
noggin (Qk034)
From the lab of Dannielle Engle, Salk Institute for Biological Studies
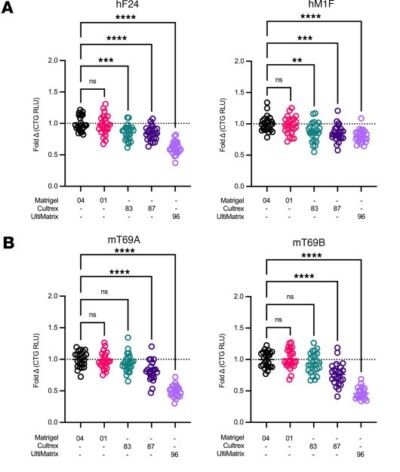
A promising application of organoids, in this case patient-derived organoids (PDOs), is to profile the therapeutic sensitivity of tumors to inform clinical decisions and to stratify patients in clinical trials more effectively. Recently there have been positive results from clinical trials giving a glimmer of the potential impact of these technologies. However, a concern is how reproducible these technologies are, particularly as most still rely on animal-derived basement membrane extracts, where batch variation is a sector-wide concern. This recent publication shows consistent drug responses are observed when different sources of commercial BME were compared. This is reassuring and speaks to how robust these approaches can be.
