Application Notes
CytoBoost™ for increased proliferation of muscle and fat cells in serum-free cultures
In this collaborative application note CytoBoost™ formulations, Perform and Maximise are tested on the proliferation of myoblasts (muscle cells) and adipocytes (fat cells) in serum-free conditions with Qkine high quality growth factors IGF-1 (Qk047), HGF NK1 (Qk061) and thermostable FGF-2, FGF2-G3 (Qk052).
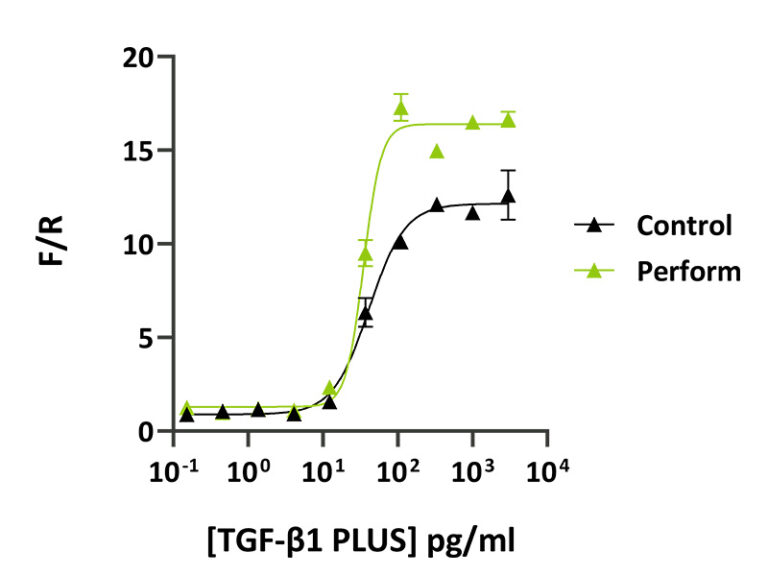
CytoBoost™-enhanced growth factor activity for cell culture and bioprocessing
In this collaborative application note CytoBoost™ formulations, Perform and Maximise are tested for their impact on the bioactivity of Qkine high quality growth factors TGF-β1 PLUS™ (Qk010), TGF-β3 (Qk054), bovine/porcine FGF-2 (Qk056), IGF-1 (Qk047), EGF (Qk011) and thermostable FGF-2, FGF2-G3 (Qk053).
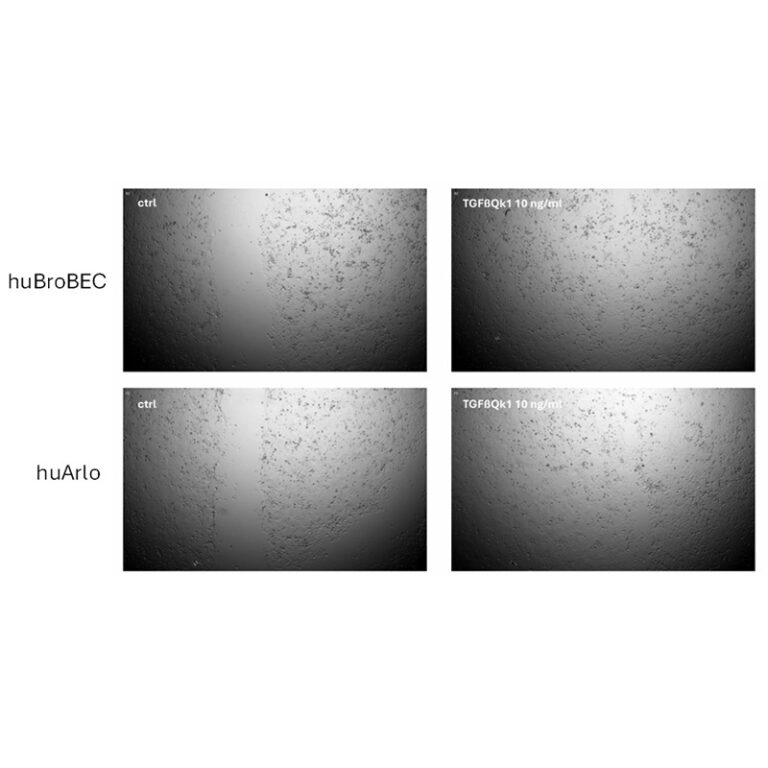
Differential induction of fibrotic signaling pathways in three immortalized lung cell lines with TGF-β1-3
This application note outlines the development and testing of de novo respiratory-related cell lines from inscreenex (bronchial, alveolar epithelial cells and lung fibroblasts). These cell lines exhibit unlimited proliferation and maintain a primary cell-line phenotype, using Qkine TGF-β1 (Qk010), TGF-β2 (Qk072) and TGF-β3 (Qk054) to stimulate the expression of lung fibrosis markers.

Differentiation of induced pluripotent stem cells (iPSCs) into dopaminergic neurons
Differentiation of induced pluripotent stem cells (iPSCs) into dopaminergic neurons requires specific conditions to obtain the desired cell type. This application note describes the method we use to differentiate human iPSCs into functional dopaminergic neurons, using a variety of media types that utilize a series of Qkine growth factors; FGF8a (Qk059), BDNF (Qk050), GDNF (Qk051) and TGF-ß3 (Qk054) at different stages of key differentiation steps.

Differentiation of induced pluripotent stem cells (iPSCs) into endoderm
Differentiating iPSCs into endodermal linage cells is particularly important due to the critical roles endodermal derivatives play in human physiology. Understanding the differentiation process of iPSCs into endodermal cells offers promise for developing treatments for a variety of conditions, including liver diseases, diabetes, and cystic fibrosis, as well as for generating organoids for research and transplantation purposes.
In this application note, we outline the method for differentiating iPSCs into definitive endoderm cells using media containing members of our recombinant FGF-2 family, such Qkine FGF2-G3 (Qk053), along with both activin A (Qk001), and BMP-4 (Qk038) growth factors.

Differentiation of induced pluripotent stem cells (iPSCs) into mesoderm
Differentiating iPSCs into mesoderm linage of cells is particularly significant, given the mesoderm’s role in generating a wide range of vital tissues and organs, including the heart, blood, muscles, and bones. This provides unparalleled potential for disease modeling, drug discovery, and cell-based therapies.
In this application note, we outline the method for differentiating iPSCs into mesoderm cells using media containing members of our recombinant FGF-2 family, such as Qkine FGF2-G3 (Qk053), along with both activin A (Qk001), and BMP-4 (Qk038) growth factors.

Differentiation of induced pluripotent stem cells (iPSCs) into microglia
In this application note, we demonstrate that high purity, animal origin-free growth factors from Qkine – including BMP-4 (Qk038), FGF2-G3 (Qk052), SCF (Qk078), VEGF 165 (Qk048), IL-3 (Qk090), M-CSF (Qk075), Flt3 ligand (Qk087), IL-34 PLUSTM (Qk091) and GM-CSF (Qk076) – support robust and reliable differentiation of iPSCs into microglial cells.

Differentiation of induced pluripotent stem cells (iPSCs) into neuroectoderm
Differentiating iPSCs into ectoderm lineage is particularly significant, given the ectoderm’s role in generating the central and peripheral nervous systems, sensory organs, and skin. Differentiating iPSCs into neuroectodermal cells, followed by further differentiating into ectodermal lineages, is critical for studying neurodevelopmental processes, modeling neurological disorders, and developing potential therapies for conditions impacting the nervous system and skin.
In this application note, we outline the method for differentiating iPSCs into neuroectoderm cells using media containing members of our recombinant FGF-2 family, such Qkine FGF2-G3 (Qk053), together with noggin (Qk034).

Maintenance of iNeurons using Qkine animal origin-free BDNF
It is essential to use high-quality and biologically active recombinant proteins to maintain the neural phenotype of iNeurons. This application note reports on the use of high purity, animal origin-free BDNF (Qk050) for the maintenance of iNeurons.

Species matched growth factors for cellular agriculture
Cultivated meat companies are being strongly advised to use growth factors with protein sequences that are 100% homologous to the species of animal they are cultivating in the production process. Growth factors from the target species also aid consumer acceptance and species-specific activity as well as regulatory considerations.
Qkine is dedicated to supporting the cellular agriculture industry by manufacturing high-purity and bioactive growth factors that are species-specific to cultivated cultures including porcine, bovine and fish.

Species-specific and optimized growth factors for the production of bovine and porcine cultivated meat and fat
Cellular agriculture has the potential to transform food production and impact the health and well-being of future generations. This application note discusses growth factors required for cultivated meat, fish and dairy and the need for species-specificity and quality, with a particular focus on FGF-2, TGF-β3 and HGF.

Stability of Qkine recombinant growth factors and cytokines in conditioned media
FGF-2 is a key component of stem cell media, it is known to be unstable under cell culture conditions, leading to the necessity for daily media changes to maintain culture conditions for stem cell culture. Although the thermo-instability of FGF-2 is well documented, the thermostability of other essential growth factors has not been investigated. This application note reviews the stability of Qkine growth factors (GM-CSF, IL-6, IGF-1, IGF1 LR3, GDNF, BMP-4, HGF NK1 and TGF-ß1 PLUS) under cell culture-like conditions to ensure maximum cost effectiveness and reproducibility in stem cell cultures.

Weekend-free human induced pluripotent stem cell culture using thermostable FGF-2 (bFGF) and animal origin-free TGF-β1 and vitronectin for improved colony homogeneity
In this application note, we describe the method for maintaining, growing, and testing the pluripotency of iPSC using E8-like media containing Qkine FGF2-G3 (Qk053) and TGF-β1 PLUS (Qk010) growth factors and using Qkine vitronectin (Qk120) as the extracellular matrix for cell attachment. This process showcases the maintenance, expansion, and pluripotency evaluation of iPSC without the need for daily feeding, enabling weekend-free maintenance and expansion while preserving the cells’ pluripotency and proliferative potential.
