The differentiation of feeder-free human induced pluripotent stem cells (iPSCs) into mesodermal cells is a complex process that uses growth factors such as activin A, BMP4, and FGF2. Additionally, factors such as CHIR99021 and the phosphatidylinositol 3-kinase (PI3-kinase) inhibitor LY294002 are required to improve the efficiency of differentiation and stabilize the mesodermal fate. This combination allows researchers to reliably generate functional mesoderm cells for various biomedical applications.
In this application note, we outline the method for differentiating iPSCs into mesoderm cells using media containing members of our recombinant FGF2 family, such as Qkine FGF2-G3 (Qk053), along with both activin A (Qk001), and BMP-4 (Qk038) growth factors. Mesoderm marker expression is used to assess successful differentiation via immunocytochemistry.
Introduction
Human induced pluripotent stem cells (iPSCs) are an in vitro model that represent a pivotal breakthrough in regenerative medicine and cellular biology. iPSCs are generated by reprogramming adult somatic cells to a pluripotent state through the introduction of specific transcription factors. Reprogramming iPSCs grants these cells the ability to differentiate into any cell type of the three germ layers: ectoderm, mesoderm, and endoderm. This provides unparalleled potential for disease modeling, drug discovery, and cell-based therapies, all without the ethical concerns associated with using embryonic stem cells [1].
Differentiating iPSCs into mesoderm linage of cells is particularly significant, given the mesoderm’s role in generating a wide range of vital tissues and organs, including the heart, blood, muscles, and bones [2].
The differentiation process of iPSCs into mesodermal cells typically involves mimicking the stages of embryonic development in vitro. During embryogenesis, mesoderm formation is initiated by a series of signaling pathways that include nodal, Wnt, and bone morphogenetic protein (BMP), which are essential for mesendoderm specification and subsequent mesoderm development [3]. To mimic these conditions in a controlled laboratory environment, researchers utilize specific growth factors and small molecules to guide iPSCs through comparable developmental signals.
The successful differentiation of iPSCs into mesodermal cells is not without challenges. Due to variability within iPSC lines, differentiation efficiencies, and the potential for incomplete or mixed lineage differentiation are significant hurdles that researchers continue to address. The success can be evaluated by investigating the expression of transcription factor markers such as brachyury and MIXL1 [4].
Materials and Methods
Cell culture and maintenance
iPSCs were passaged twice per week using 0.5 mM EDTA for detachment and seeded in vitronectin (Qk120) (5 µg/ml) coated 6-well plates using a 1:6 split ratio and cultured in an E8-like media. The day after passage, spent media was removed to be replaced with 5 ml of E8-like media allowing the cells to follow a weekend-free media change pattern. For further information on this process, please see our guide to Weekend-free human induced pluripotent stem cell culture using thermostable FGF-2 (bFGF) from Qkine, together with our animal origin-free TGF-β1 and vitronectin, for improved colony homogeneity.
iPSC differentiation into mesoderm
The mesoderm differentiation workflow schematic (figure 1) outlines the steps for the differentiation of iPSCs into mesoderm and assessing differentiation by testing mesoderm expressing markers.
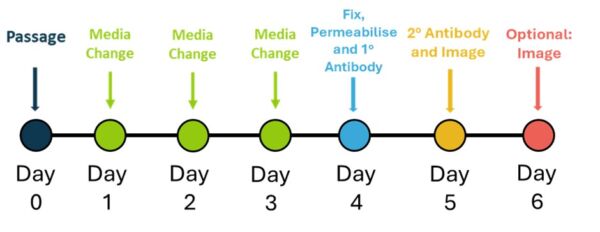
Figure 1. Schedule for Mesoderm differentiation and evaluation testing
iPSCs were detached using AccutaseTM and seeded at 1,000 cells / well in a vitronectin (Qk120) (5 µg/ml) coated 96-well plate in E8-like media containing ROCK inhibitor (Y-27632, 10 µM). The following day, Day 1, cells were fed with freshly prepared mesoderm medium (Table 1). Media change on days 2 and 3, using freshly prepared mesoderm medium.
| Media and supplements | Mesoderm medium |
|---|---|
| CDM-PVA (Table 2) | Base |
| Recombinant Human activin A (Qk001) | 10 ng/ml |
| Recombinant FGF-2 (Qk053) | 20 ng/ml |
| Recombinant BMP-4 (Qk038) | 10 ng/ml |
| LY294002 (Promega V1201) | 10 µM |
| CHIR99021 (Stratech S2924-SEL) | 16.6 µM |
Table 1. Daily mesoderm medium construction components. Components added aseptically before use.
| CDM-PVA medium | |
|---|---|
| F-12 Nutrient Mix (Thermo Scientific 31765027) | Half of base media |
| IMDM (Fisher Scientific 11510596) | Half of base media |
| 5% PVA solution [5 g PVA (Merck P8136) in 100 ml water from embryo transfer (Merck W1503)] | 0.1% |
| CD concentrated Lipids (Thermo Scientific 11905031) | 1% |
| Transferrin (Merck T1147) | 15 µg/ml |
| 1-Thioglycerol (Merck M6145) | 0.5 mM |
Table 2. CDM-PVA medium construction components. Components added aseptically and media filtered before use.
Immunocytochemistry
On Day 4, cells were fixed with 4% paraformaldehyde, blocked and permeabilized with 10% donkey serum diluted in 0.1% Triton X-100. Specific antibodies for mesodermal expression Brachyury and MixL1 were applied for immunostaining overnight at 4°C. iPSCs were then washed and incubated with the secondary antibodies Donkey anti-Goat AlexaFluorTM 488 or Donkey anti-Rabbit AlexaFluorTM 488 and Hoechst 33258, followed by imaging in phosphate buffered saline.
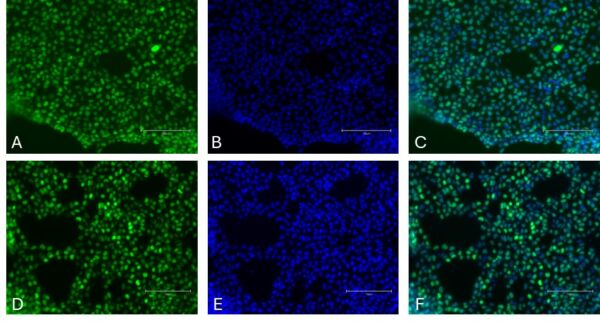
Figure 2. Immunocytochemistry of Mesoderm markers in differentiated iPSCs. Transcription factor Brachyury [Green, A], Hoechst 33258 [Blue, B], combined Brachyury and Hoechst [C] and transcription factor MIXL1 [Green, D], Hoechst33258 [Blue, E], combined MIXL1 and Hoechst [F]. Images were acquired using the EVOS M5000 (scale bar = 150 µm).
Conclusion
The differentiation of iPSCs into mesodermal cells is an evolving yet highly promising field that holds significant therapeutic potential. By leveraging our understanding of embryonic development and refining differentiation protocols, allow researchers to reliably generate functional mesodermal cells for use in a wide range of biomedical applications.
The data presented in this application note demonstrate that using Qkine FGF2-G3, Qkine activin A and BMP-4 support the differentiation of iPSCs into mesoderm cells.
Further Information
Qkine growth factors are manufactured to the highest of quality standards and are free from animal-derived contaminants, delivering low endotoxicity and high purity. At Qkine, we are committed to raising the standards of growth factors, cytokines and related proteins to better support long-term and complex neural stem cell culture. We are a science-led team, please reach out with any questions or requests to support@qkine.com.