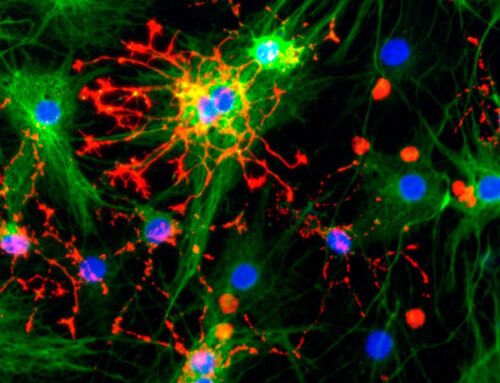
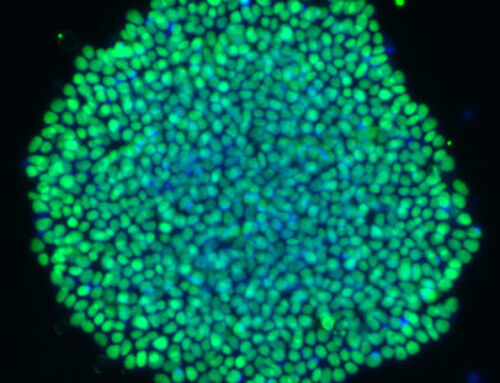
Hepatocyte-derived organoids provide unique insights into liver cell invasion by malaria parasites
Complex 3D liver models that more closely mimic human biology open opportunities for improved drug and vaccine discovery for malaria and related parasitic diseases
Image credit: NIAID
Malaria is a major global socioeconomic burden and presents unique challenges for the scientific community
Malaria is a parasitic disease caused by the protozoan parasite Plasmodium falciparum. It is one of the major global infectious diseases and a priority for WHO with over 240 million new cases of malaria being diagnosed each year and over 600,000 deaths. The greatest number of cases and most socioeconomic impact is felt in Sub-Saharan Africa, where the majority of deaths, >80%, sadly occur in children under five.
The development of new malaria drugs and vaccines is scientifically challenging. Current disease models often rely on mouse infection models, and although preserved primary hepatocytes have been used to establish more human-relevant models, these often have low permissibility for parasite invasion and do not support the complete life cycle. Recently, complex liver organoid models have shown promise for identifying improved malarial targets for intervention. It is intriguing to consider whether a vascularised or organ-on-chip model may also soon be developed which could support the entire parasite life-cycle – a fascinating concept.
Despite global investment in malaria research over the last decade, including by organizations such as the Gates Foundation, Wellcome Trust, and the World Health Organization (WHO), malaria remains a scientifically challenging disease to study. Genome sequencing of parasites, the vector anopheline mosquitos, and human hosts has provided insights into biology and a unique perspective on the rapid evolution of the parasite. This contributes to the rapid evolution and spread of drug resistance to particularly artemisinin-based drugs, one of the only effective therapies. In addition, despite intense global efforts, developing effective vaccines is immensely challenging due to the fundamental biology of the parasite and host-parasite interactions.
Only one vaccine for malaria, RTS,S, targeting a surface antigen on P. falciparum, has been recommended for use by the WHO in childhood immunization programs. A full course of vaccination in under-fives reduces the number of lifetime infections and the severity of life-threatening malaria. This modest efficacy against malaria illness, combined with the need for at least four immunizations illustrates the remaining pressing need for the R&D community to identify new vaccine and drug targets.
Liver organoids provide new biologically relevant models for malaria drug discovery
The complex multi-stage P. falciparum life cycle, combined with rapid evolution and agile host immune evasion mechanisms, presents a unique challenge to researchers trying to identify new druggable targets and vaccine candidates.
Although recent advances in single-cell sequencing and mass genome sequencing have propelled the field forward, existing biological models have severe limitations. Many studies rely on mouse models with the related parasite Plasmodium berghei, which poorly recapitulate the complex interplay of human parasite-host responses, meaning there is a pressing need for more human-relevant models.
Cryopreserved primary hepatocytes have been used to create in vitro human models, however, these are challenging to culture for long periods and often have low permissibility for parasite invasion. Similarly, hepatocyte immortalized cell lines often exhibit variable permissibility and perturbation of early stages of parasite invasion.
There is a pressing need for a human in vitro model with sufficient complexity and longevity to improve success of identifying true targets for intervention and in parallel reduce the reliance on animal models.
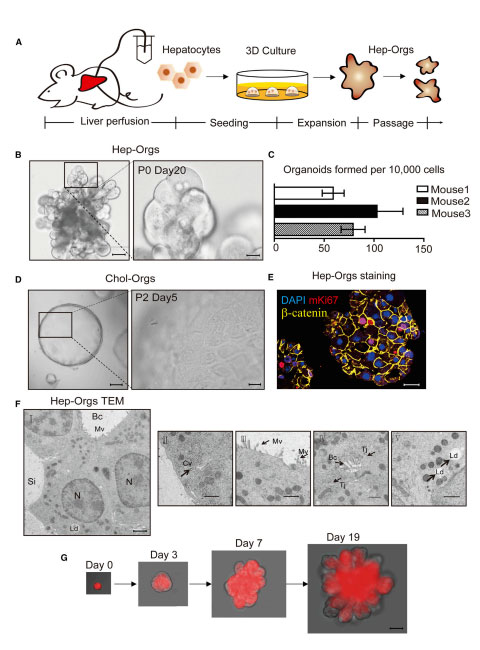
Figure 1. Source: https://doi.org/10.1016/j.cell.2018.11.013
Human proliferative hepatocyte organoids developed by HUB
Recently, Hu et al, from the Hubrecht Institute developed proliferative hepatocyte organoids (Hep-Orgs), distinct from cholangiocyte derived organoids (Chol-Orgs).
Building on this work, an elegant study by Yang et al was conducted in a collaboration between Sauerwein lab at Radboud Center of Infectious Diseases, Radboud University Medical Center, and Hubrecht Institute, Royal Netherlands Academy of Arts and Sciences, The Netherlands. Well-characterised defined hepatocyte organoids were used to give a unique insight into life cycle of the Plasmodium parasite in host cells and was combined with single cell transcriptomics to generate an informative non-biased dataset.
As an example of utility, the authors highlight the discovery of a later functional role in parasite development for SR-B1, already known to be involved in early phases. Intriguingly, this unique window on biological mechanism appeared parasite strain independent, suggesting that liver organoid model systems, combined with transcriptomics, have promise for the elucidation of new malarial drug targets and opportunities for determination of drug mechanism.
The next steps for this technology, and a challenge many organoid platforms are facing, is how to effectively scale the technology to establish an organoid-based drug target identification platform. As we see these systems scale and become standardized, this opens opportunities to build further biological complexity into organoid models and deploy CRISPR to begin to develop an understanding of human hepatocyte host cell factors.
Figure 1. Establishment of 3D Culture System of Murine Hepatocyte Organoids
(A) Schematic depicting the isolation and seeding of primary hepatocytes, and the expansion and passage of Hep-Orgs.
(B) Differential interference contrast (DIC) images of Hep-Orgs at Passage 0 (P0) day 20 cultured from primary hepatocytes of wild-type C57BL/6 mice. Lowermagnification (left, black scale bar = 50 mm), Higher magnification (right, black scale bar = 12.5 mm).
(C) Numbers of organoids formed per 10,000 hepatocytes. Experiments were performed in triplicate and on independent C57BL/6 mice. Data are represented asmean ± SEM.
(D) Differential interference contrast (DIC) images of Chol-Orgs at Passage 2 day 3 (left, black scale bar = 50 mm); Higher magnification (right, black scalebar = 12.5 mm).
(E) Confocal image of paraffin section of a Hep-Org co-stained for proliferation marker mKi67 (Red), the adhesion junction marker b-catenin (yellow) and DAPI(blue). Scale bar = 25 mm.
(F) Transmission EM (TEM) of Hep-orgs shows typical hepatocyte structures.
(G) A clonal Hep-Org grown from a single primary hepatocyte (tdTomato+) from atamoxifen-induced Albumin-CreERT2; Rosa 26-LSL-tdTomato mouse. Scale bar=25mm.
Purchase growth factors for organoids in bulk & save
We offer scalable recombinant proteins, which are available in bulk and in custom sizes to suit your requirements. Contact us today to discuss your needs and for additional discounts on bulk purchases.
A member of our team will come back to you shortly to discuss pricing and availability.
Purchase growth factors for organoids in bulk & save
We offer scalable recombinant proteins, which are available in bulk and in custom sizes to suit your requirements. Contact us today to discuss your needs and for additional discounts on bulk purchases.
A member of our team will come back to you shortly to discuss pricing and availability.