Currency
Recombinant FGF2-G3 (145 aa) protein (Qk052)
FGF2-G3 (145 aa) protein is a thermostable engineered form of FGF-2 (bFGF). Qk052 comprises the 145 aa form of FGF-2 (Qk025) with the nine amino acid substitutions developed by Dvorak et al. 2018. This increases the functional half-life of the protein from <10 h (wild-type) to >7 days (FGF2-G3 145 aa).
FGF2-G3 is used in B8 media (Kuo et al. 2019) for weekend free, high homogeneity induced pluripotent stem cell culture. This new 145 aa thermostable FGF-2 protein allows direct comparison of efficacy in systems using FGF-2 145 aa (Qk025) for chemically defined stem cell and organoid culture media, and cultured meat media development.
High purity 16 kDa bioactive FGF2-G3 145 aa protein, animal origin-free (AOF), carrier protein-free and with no His-tag.
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
1000µg will be despatched as 2 x 500µg
Fast and free shipping.
Buy online with secure credit card or purchase order.
For any questions, please email orders@qkine.com
Summary
High purity thermostable FGF2-G3 protein comprising 145 aa form of FGF-2 (Uniprot: P09038) with nine stabilizing amino acid substitutions
>98%, by SDS-PAGE quantitative densitometry
16 kDa
Expressed in E. coli.
Animal origin-free (AOF) and carrier protein-free.
Manufactured in our Cambridge, UK laboratories
Lyophilized from Tris/NaCl/CyS/mannitol
Resuspend in water at >100 µg/ml, prepare single use aliquots, add carrier protein if desired, store frozen at -20 oC (short-term) or -80 oC (long-term)
Featured applications
Expansion of induced pluripotent, embryonic and mesenchymal stem cells
Cell expansion
Species neutral
Alternative proteins
Recombinant human FGF-2 (145 aa) protein (Qk025): wild-type form of this protein
Recombinant FGF-G3 (154 aa) protein (Qk053): 154 aa form of hyperstable FGF2-G3
Recombinant human FGF-2 (154 aa) protein (Qk027)
Recombinant zebrafish FGF-2 protein (Qk002)
Related discovery kits
This protein has recently been reformulated and will now arrive lyophilized. This change has been made to provide enhanced stability to ensure that we offer the best customer experience in terms of shipping and storage and to align with our company sustainability goals. Please visit our reconstitution page or contact customerservice@qkine.com for further information or to request the previous datasheet.
Bioactivity
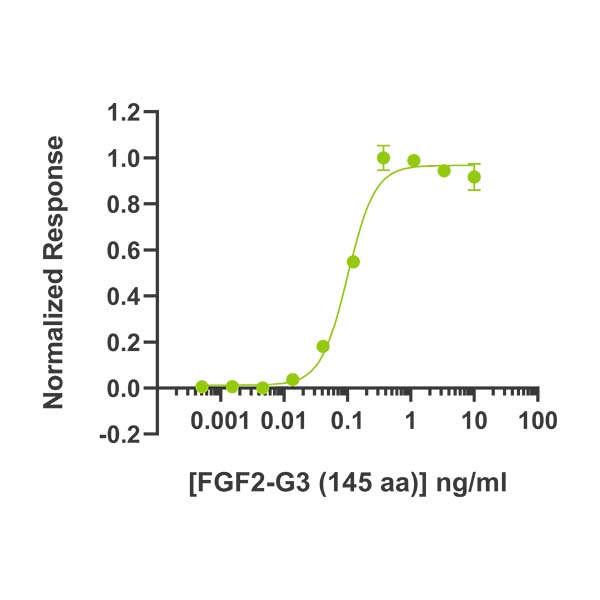
FGF2-G3 (145 aa) activity is determined using the Promega serum response element luciferase reporter assay (*) in transfected HEK293T cells. EC50 = 101 pg/ml (6.2 pM). Cells are treated in triplicate with a serial dilution of FGF2-G3 (145 aa) for 3 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. Data from Qk052 lot #104339. *Promega pGL4.33[luc2P/SRE/Hygro] #E1340
Purity
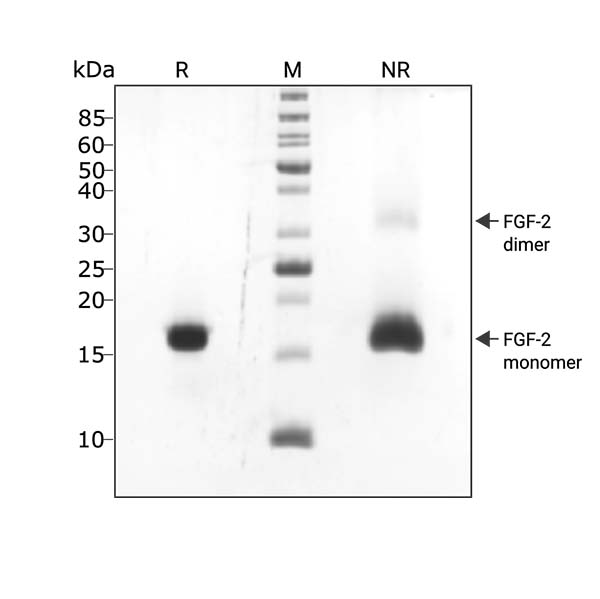
FGF2-G3 (145 aa) migrates as a major band at 16 kDa in non-reducing (NR) conditions. A small amount of dimer can be seen (higher molecular weight band). Upon reduction (R), only the 16 kDa band is visible. No contaminating protein bands are present. Purified recombinant protein (3 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced (NR) conditions and stained with Coomassie Brilliant Blue R250. Data from Qk052 batch #104339.
Further quality assays
Mass spectrometry: single species with expected mass
Analytical reversed-phase: single sharp peak
Endotoxin: <0.005 EU/μg protein (below level of detection)
Recovery from stock vial >95%
We are a company founded and run by scientists to support innovation in stem cell biology and regenerative medicine. To enhance reliability and reproducibility in your applications, all our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Product manufactured under license: International Patent Application No. PCT/EP2016/073567 and U.S. Patent No. 11,746,135
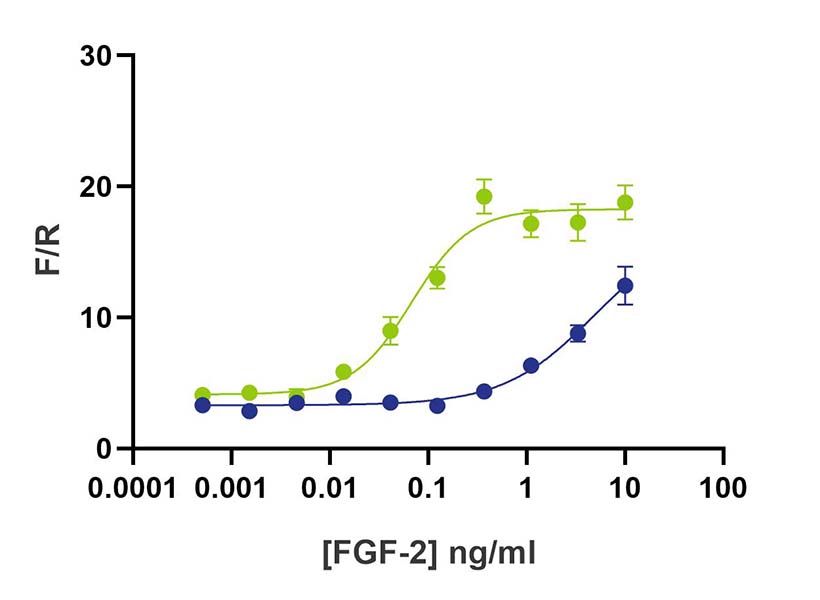
Wild-type FGF-2 (145 aa) and FGF-G3 (145 aa) have equivalent bioactivity

FGF2-G3 (145 aa) activity is determined using the Promega serum response element luciferase reporter assay (*) in transfected HEK293T cells. Cells are treated in triplicate with a serial dilution of FGF2-G3 for 3 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. *Promega pGL4.33[luc2P/SRE/Hygro] #E1340
‘After a long day running samples and analysing data, it was very exciting to see just how stable the FGF2-G3 proteins were. They clearly have more endurance than me!’ – Kerry, Qkine Senior Scientist
Hyperstable FGF2-G3 (145 aa) retains activity after pre-incubation with conditioned media at 37o C
+24 hours
+48 hours

+72 hours
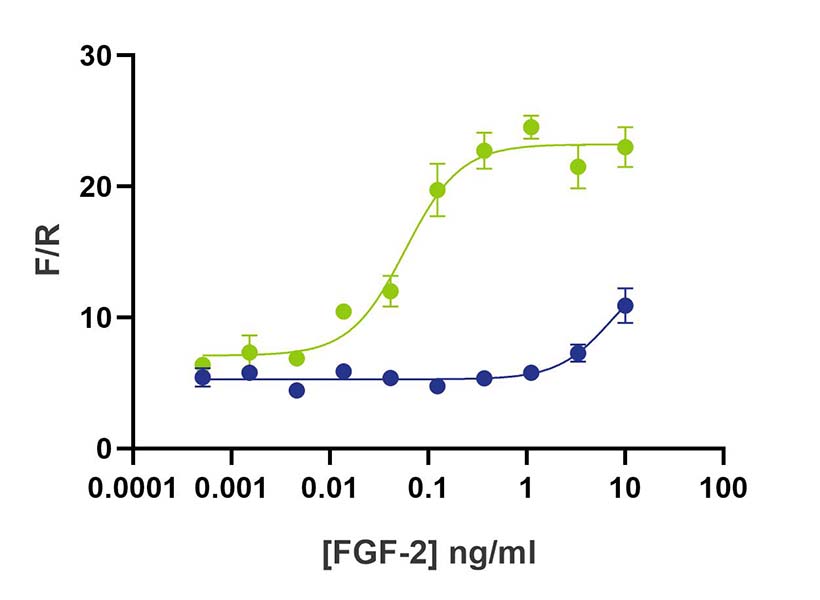
FGF2-G3 145 aa (Qk052) WT FGF2 145 aa (Qk025)
WT FGF2 (Qk025) and FGF2-G3 (Qk052) were diluted in conditioned media and incubated at 37oC. Samples were taken at daily intervals, serially diluted and then assayed in triplicate for activity using the Promega serum response element luciferase reporter assay (*) in transfected HEK293T cells. Firefly luciferase activity was measured and normalized to the control Renilla luciferase activity (F/R).
FGF2-G3 protein background
FGF-2 (also known as basic FGF or bFGF) is an essential growth factor for maintaining human embryonic stem cell (hESC) and induced pluripotency stem cell (iPSC) pluripotency in feeder-free and chemically defined stem cell media. It is a core component of widely adopted media including mTESR (Ludwig et al. 2006), StemPRO (Wang et al. 2007) and E8 (Chen et al. 2011). However, FGF-2 is inherently unstable and prone to proteolytic degradation and aggregation. This fundamental biochemical instability, and therefore low half-life in culture media (<10 h), is an important contribution to the need for frequent media changes and challenges in improving homogeneity during stem cell proliferation and subsequent differentiation.
To improve the stability of FGF-2 for stem cell media and regenerative medicine applications, Dvorak and colleagues at Masaryk University used computer-assisted protein engineering to identify an optimal set of nine amino acid substitutions that stabilize FGF-2. These substitutions were designed to avoid structural changes to the FGF receptor 1 (FGFR1) and FGF receptor 2 (FGFR2) binding sites. This thermostable FGF-2 is known as FGF2-G3, or FGF2-STAB® (Dvorak et al. 2017). The biological activity of wild-type FGF-2 is <50% after 10h incubation with conditioned media. In contrast, no reduction in FGF2-G3 biological activity is observed after >7 days incubation with conditioned media at 37oC. Both FGF2-G3 and wild-type FGF-2 maintain hESC pluripotency and expression of pluripotency markers Oct-4 and nanog with equivalent efficacy (Dvorak et al. 2017).
In 2020, Paul Burridge and colleagues at Northwestern University, Chicago, published a protocol for B8 media. This iPSC maintenance media uses thermostable FGF2-G3, along with optimization of media component concentration and composition to reduce media cost and facilitate weekend-free stem cell culture regimes. (Kuo et al. 2020 and updated in Lyra-Leite et al. 2020).
To manufacture and provide FGF2-G3 for stem cell culture, including use in B8 media and emerging applications such as cultured meat, Qkine has licensed the patented FGF2-G3 technology from Enantis/Masaryk University. We have combined the excellent science behind the FGF2-G3 technology with our protein manufacture expertise to provide gold standard protein for use in cell culture media. We have removed His tags present in academic forms of the protein, as these may give rise to issues for scientists translating discoveries to the clinical or scale-up. His tags also introduce unnecessary scientific uncertainty.
Two forms of FGF-2 are used frequently in stem cell culture: 145 aa (Qk025) and 154 aa (Qk027).
To allow scientists to compare directly with their existing FGF-2, we have introduced the nine amino acid substitutions into the 145 aa form of FGF-2 to produce Qk052 FGF2-G3 (145 aa). The EC50 of the two forms is equivalent and the half-life is extended from less than 1 day to >3 days.
Additional resources
FGF2-G3 Key Papers
Publications using FGF2-G3 (145 aa) protein (Qk052)
Refined home-brew media for cost-effective, weekend-free hiPSC culture and genetic engineering.
In Open Research Europe 2024 by Truszkowski, L., Bottini, S., Bianchi, S. et al.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.



What others are saying
There are no contributions yet.