 Recombinant human gremlin 1 protein (Qk015)
Recombinant human gremlin 1 protein (Qk015)Recombinant human gremlin 1 protein (Qk015)
Price range: £175.00 through £880.00
Human gremlin 1 protein is a BMP-inhibitor present in the natural intestinal niche and provides an alternative to noggin for optimization of intestinal organoid culture and iPSC differentiation.
Qkine gremlin 1 has been optimized by our experts to be an exceptionally high-purity 18 kDa highly bioactive dimeric protein, animal origin-free (AOF) and carrier protein free.
In stock
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
Price range: £175.00 through £880.00
Buy online with secure credit card or purchase order. For any questions, please email orders@qkine.com
Summary:
- High purity human gremlin 1 protein (Uniprot: O60565)
- 18 kDa
>98%, by SDS-PAGE quantitative densitometry
Expressed in E. coli
Animal origin-free (AOF) and carrier protein-free
Manufactured in our Cambridge, UK laboratories
Lyophilized from acetonitrile, TFA
- Resuspend in 10 mM HCl (Reconstitution solution A) at >50 µg/ml, add carrier protein if desired, prepare single-use aliquots and store frozen at -20 °C (short-term) or -80 °C (long-term)
Featured applications:
Tumor organoid culture
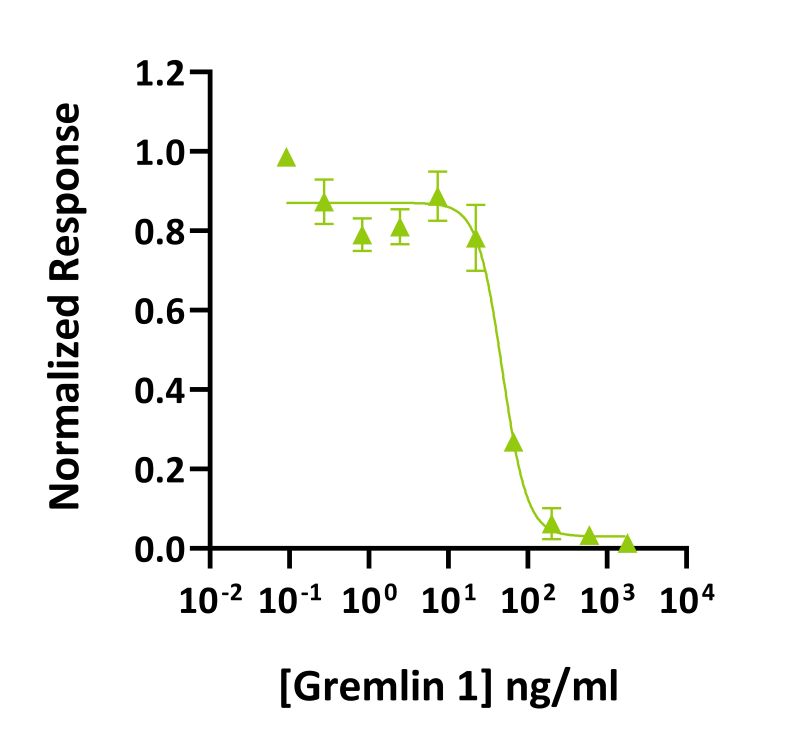
Gremlin-1 inhibits BMP-2 induced luciferase activity BRE-HEK293 luciferase reporter assay with an IC50 = 34 ng/ml (1.88 nM). Gremlin 1 activity was determined using inhibition of the BMP-2 response (Qk007 #010, 52 ng/ml) from a BMP2-responsive firefly luciferase reporter in stably transfected HEK293T cells. Cells were treated (n=4) with a serial dilution of gremlin 1 in BMP-2 for 6 hours. Firefly luciferase activity was measured and normalized to the control Renilla luciferase activity. Data from Qk015 batch #011.
Gremlin 1 protein migrates as a single diffuse band at ~36 kDa in non-reducing (NR) and 19 kDa in reducing (R) conditions. The protein is a non-covalent dimer and it is the dissociation of the dimer during electrophoresis which gives the characteristic diffuse band. Purified recombinant protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250. Data from Qk015 lot #011.

Further quality assays
Mass spectrometry: single species with expected mass
Recovery from stock vial: >95%
Endotoxin: <0.05 EU/μg protein
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
Gremlin 1 protein (GREM1, isoform-1) belongs to the bone morphogenetic protein (BMP) antagonist family. Gremlin 1 protein binds BMP-2, BMP-4, BMP-7 and other BMP family proteins [1] and inhibits receptor binding. It is highly expressed in the small intestine at the base of the intestinal crypts, as are the related proteins, gremlin 2 and chordin-like 1. In this niche, they help maintain the stem cell population by inhibiting BMP produced by mesenchymal cells [2]. Expression of gremlin 1 protein is also detected in fetal brain and colon, and at lower levels in adult brain, prostate, pancreas and skeletal muscle.
Recombinant human gremlin 1 protein and other BMP-antagonists, such as noggin, are used in the derivation, growth and maintenance of organoids from epithelial tissues including intestinal, liver and pancreatic organoids. However, roles for recombinant gremlin 1 protein in cancer stem cell maintenance in glioblastoma have been suggested [3].
Additional resources
Publications using Recombinant human gremlin 1 protein (Qk015)
-
Basal delamination during mouse gastrulation primes pluripotent cells for differentiation
Sato N, Rosa VS, Makhlouf A et al.
DOI: doi: 10.1016/j.devcel.2024.03.008
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.
For use in manufacturing of cellular or gene therapy products. Not intended for in vivo applications.

Receive an Amazon gift voucher when you leave us a review.
£25, $30 or €30 for reviews with an image and £10, $15 or €15 for reviews without an image

