Differentiation of induced pluripotent stem cells (iPSCs) into neuroectoderm
Leighton Sneade
Qkine
The differentiation of feeder-free human induced pluripotent stem cells (iPSCs) into neuroectodermal cells is a complex process that uses growth factors such as noggin and FGF2. Additionally, factors such as activin receptor-like kinase receptor blocker SB431542 are required to improve the efficiency of differentiation and stabilize the neuroectodermal fate. This combination allows researchers to reliably generate functional neuroectodermal cells for various biomedical applications. In this application note, we outline the method for differentiating iPSCs into neuroectoderm cells using media containing members of our recombinant FGF2 family, such Qkine FGF2-G3 (Qk053), together with noggin (Qk034). Neuroectoderm marker expression is used to assess successful differentiation via immunocytochemistry.
Introduction
Human induced pluripotent stem cells (iPSCs) are an in vitro model that represent a pivotal breakthrough in regenerative medicine and cellular biology. iPSCs are generated by reprogramming adult somatic cells to a pluripotent state through the introduction of specific transcription factors. Reprogramming iPSCs grants these cells the ability to differentiate into any cell type of the three germ layers: ectoderm, mesoderm, and endoderm. This provides unparalleled potential for disease modeling, drug discovery, and cell-based therapies, all without the ethical concerns associated with using embryonic stem cells [1].
Differentiating iPSCs into ectoderm lineage is particularly significant, given the ectoderm’s role in generating the central and peripheral nervous systems, sensory organs, and skin [2]. Differentiating iPSCs into neuroectodermal cells, followed by further differentiating into ectodermal lineages, is critical for studying neurodevelopmental processes, modeling neurological disorders, and developing potential therapies for conditions impacting the nervous system and skin.
The differentiation of iPSCs into neuroectodermal cells typically involves mimicking the stages of embryonic development in vitro. During early embryogenesis, the formation of neuroectoderm is initiated by inhibiting several signaling pathways, such as the TGF-β pathway using small molecule inhibitors like SB431542, and the bone morphogenetic protein (BMP) pathway using BMP inhibitors like noggin, which mimics the natural inhibition of BMPs that occurs during neural plate formation [3].
The successful differentiation of iPSCs into neuroectodermal cells is not without challenges. Due to variability within iPSC lines, differentiation efficiencies, and the potential for incomplete or mixed lineage differentiation are significant hurdles that researchers continue to address. The success can be evaluated by investigating the expression of markers such as SRY-box transcription factor 1 (SOX1) and SRY-box transcription factor 2 (SOX2) [4].
References
[1] Varum, S. et al. Energy Metabolism in Human pluripotent stem cells and their differentiated counterparts. PLoS ONE. 2011;6(6):e20914. doi: 10.1371/journal.pone.0020914
[2] Tchieu, J. et al. A Modular Platform for Differentiation of Human PSCs into All Major Ectodermal Lineages. Cell Stem Cell. 2017 Sep 7;21(3):399-410.e7. doi: 10.1016/j.stem.2017.08.015
[3] Smith, J. R. et al. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Developmental Biology. 2008 Jan 1;313(1):107-17. doi: 10.1016/j.ydbio.2007.10.003
[4] Galiakberova, A. A. et al. Neural Stem Cells and Methods for Their Generation From Induced Pluripotent Stem Cells in vitro. Frontiers in cell and developmental biology. 2020 Oct 8:8:815. doi: 10.3389/fcell.2020.00815
Materials and Methods
Cell culture and maintenance
iPSCs were passaged twice per week using 0.5 mM EDTA for detachment and seeded in vitronectin (Qk120) (5 µg/ml) coated 6-well plates using a 1:6 split ratio and cultured in an E8-like media. The day after passage, spent media was removed to be replaced with 5 ml of E8-like media allowing the cells to follow a weekend-free media change pattern. For further information on this process, please see our guide to Weekend-free human induced pluripotent stem cell culture using thermostable FGF-2 (bFGF) from Qkine, together with our animal origin-free TGF-β1 and vitronectin, for improved colony homogeneity.
iPSC differentiation into neuroectoderm
The neuroectoderm differentiation workflow schematic (figure 1) outlines the steps for the differentiation of iPSCs into neuroectoderm and assessing differentiation by testing neuroectoderm expressing markers.
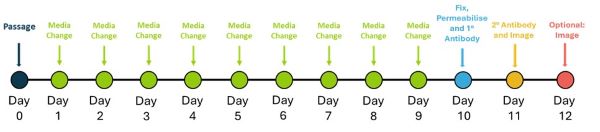
Figure 1. Schedule for neuroectoderm differentiation and evaluation testing
iPSCs were detached using AccutaseTM and seeded at 1,000 cells / well in a vitronectin (Qk120) (5 µg/ml) coated 96-well plate in E8-like media containing ROCK inhibitor (Y-27632, 10 µM). The following day (Day 1) and every day until Day 10, cells were fed with ectoderm medium (Table 1).
| Media and supplements | Neuroectoderm medium |
|---|---|
| CDM-PVA (Table 2) | Base |
| SB431542 (R&D Systems 1614/1) | 10 ng/ml |
| Recombinant FGF2-G3 (Qk053) | 12 ng/ml |
| Recombinant human noggin (Qk034) | 15 ng/ml |
Table 1. Daily neuroectoderm medium construction components. Components added aseptically before use.
Data shown from FGF2-G3 (Qk053), this protocol has also been successfully completed with recombinant zebrafish FGF-2 (bFGF) protein (Qk002), human FGF-2 145 aa (Qk025), human FGF-2 154 aa (Qk027), bovine/porcine FGF-2 145aa (Qk040), mouse FGF-2 (Qk042), FGF2-G3 145 aa (Qk052) and bovine/porcine FGF-2 154 aa (Qk056).
| CDM-PVA medium | |
|---|---|
| F-12 Nutrient Mix (Thermo Scientific 31765027) | Half of base media |
| IMDM (Fisher Scientific 11510596) | Half of base media |
| 5% PVA solution [5 g PVA (Merck P8136) in 100 ml water from embryo transfer (Merck W1503)] | 0.1% |
| CD concentrated Lipids (Thermo Scientific 11905031) | 1% |
| Transferrin (Merck T1147) | 15 µg/ml |
| 1-Thioglycerol (Merck M6145) | 0.5 mM |
Table 2. CDM-PVA medium construction components. Components added aseptically and media filtered before use.
Immunocytochemistry
On Day 10, cells were fixed with 4% Paraformaldehyde, blocked and permeabilized with 10% donkey serum diluted in 0.1% Triton X-100. Specific antibodies for ectodermal expression SOX1 and SOX2 were applied for immunostaining overnight at 4°C. iPSCs were then washed and incubated with the secondary antibodies Donkey anti-Goat AlexaFluorTM 488 and Hoechst 33258, followed by imaging in phosphate buffered saline.
Results
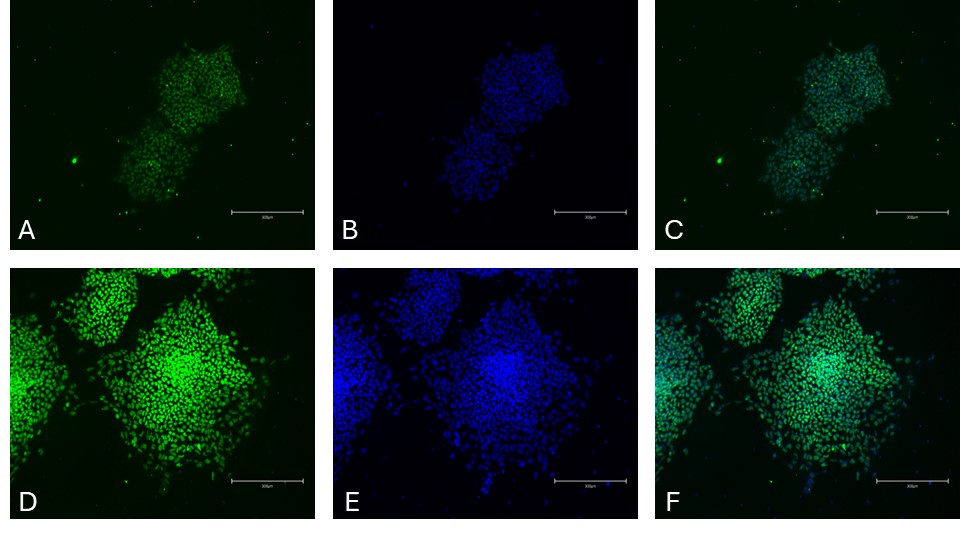
Figure 2. Immunocytochemistry of neuroectoderm markers in differentiated iPSCs. SRY-box transcription factor 1 (SOX1) [Green, A], Hoechst 33258 [Blue, B], combined SOX1 and Hoechst [C] and SRY-box transcription factor 2 (SOX2) [Green, D], Hoechst33258 [Blue, E], combined SOX2 and Hoechst [F]. Images were acquired using the EVOS M5000 system (scale bar = 300 µm).
Conclusion
The importance of iPSCs in clinical and research fields is increasing due to their potential to differentiate into all somatic cell types in the human body. The successful differentiation of iPSCs to required cell types, such as neuroectodermal cells, relies on maintaining the pluripotency of iPSCs during culture combined with homogenous differentiation. The use of correct media containing highly pure bioactive growth factors is therefore critical for reproducibility achieving this.
The data presented in this application note demonstrate that using Qkine FGF-2 and Qkine human noggin support the differentiation of iPSCs into neuroectoderm on vitronectin coated plates.
Further Information
Qkine growth factors are manufactured to the highest of quality standards and are free from animal-derived contaminants, delivering low endotoxicity and high purity. At Qkine, we are committed to raising the standards of growth factors, cytokines and related proteins to better support long-term and complex neural stem cell culture. We are a science-led team, please reach out with any questions or requests to support@qkine.com.
All our recombinant proteins are animal-free and come with Bioactivity. Guaranteed.
Contact us
Our science team is here to help, please contact us if you have any questions.