 Recombinant human FGF-8a protein (Qk059)
Recombinant human FGF-8a protein (Qk059)Recombinant human FGF-8a protein (Qk059)
£155.00 – £2,300.00

Fibroblast growth factor 8a (FGF-8a) is a member of the FGF family and plays a key role in the regulation of embryogenesis, cellular proliferation, differentiation, and migration. FGF-8a is often used for the differentiation of induced pluripotent stem cells, embryonic stem cells, and neural stem cells.
FGF-8a is a spliced form of FGF-8, a heparin-binding protein that targets mammary and non-mammary cells expressing the FGF receptors. A 21.3 kDa highly pure, bioactive recombinant protein produced in an animal-free expression system. This protein is animal-origin free (AOF), carrier protein-free, tag-free, and non-glycosylated to ensure a pure homogenous protein with exceptional lot-to-lot consistency. Qk059 is suitable for enhanced reproducibility and physiologically relevant cultures.
In stock
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
£155.00 – £2,300.00
Fast and free shipping.
Buy online with secure credit card or purchase order. For any questions, please email orders@qkine.com
Summary:
- High purity human FGF-8a protein (Uniprot: P55075-2)
- 21.3 kDa
>98%, by SDS-PAGE quantitative densitometry
Expressed in E. coli
Animal origin-free (AOF) and carrier protein-free
Manufactured in our Cambridge, UK laboratories
Lyophilized from HEPES, NaCl, mannitol
- Resuspend in sterile-filtered water at >50 µg/ml, add carrier protein if desired, prepare single use aliquots and store frozen at -20 °C (short-term) or -80 °C (long-term).
Featured applications:
Differentiation of midbrain dopaminergic neurons
Neurite outgrowth from spiral ganglion neurons
Morphogenesis of mid-hindbrain
Differentiation of iPSC derived neural progenitors to neurons

FGF-8a activity was determined using the Promega serum response element luciferase reporter assay (*) in HEK293T cells. EC50 = 110 ng/ml (5.2 nM). Cells were treated in triplicate with a serial dilution of FGF-8a in the presence of 10 µg/ml heparin for 3 hours. Firefly luciferase activity was measured and normalized to the control Renilla luciferase activity. Data from Qk059 lot #104406. *Promega pGL4.33[luc2P/SRE/Hygro] #E1340
FGF-8a (Qk059) migrates as a single band at 21.3 kDa in non-reducing (NR) conditions and upon reduction (R). Purified recombinant protein (3 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced (NR) conditions and stained with Coomassie Brilliant Blue R250. Data from Qk059 batch #104412.

Further quality assays
Mass spectrometry: single species with expected mass
Recovery from stock vial: >95%
Endotoxin: <0.005 EU/μg protein (below level of detection)
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
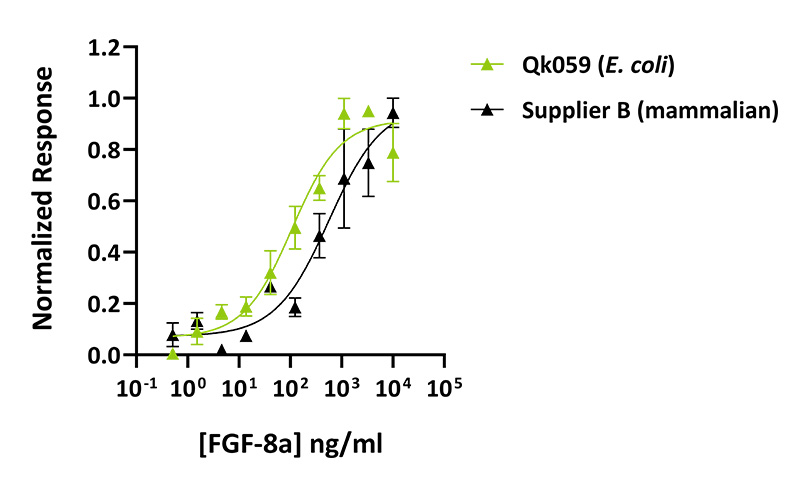
Qkine animal origin-free FGF-8a is more biologically active than mammalian-origin comparable alternative. Quantitative luciferase assay with Qkine FGF-8a (Qk059, green) and alternative supplier FGF-8a (Supplier B, black). Cells were treated in triplicate with a serial dilution of FGF-8a for 3 hours in the presence of 10 µg/ml heparin. Firefly luciferase activity was measured and normalized to control Renilla luciferase activity.
Technote | FGF-8a (Qk059) bioactivityProtein background
FGF-8a is a heparin-binding protein and an isoform of FGF-8 belonging to a family of fibroblast growth factors (FGF) [1]. It was originally discovered as an essential growth factor for the androgen-dependent growth of mouse mammary carcinoma cells [2]. In mouse, there are eight spliced protein isoforms of FGF8 (a-h) whereas, in humans, there are four alternate spliced protein isoforms namely FGF-8a, FGF-8b, FGF-8e, and FGF-8f. These four FGF8 isoforms (a, b, e and f) are highly conserved between humans and mice. Human and murine FGF-8a and FGF-8b show 100% homology [3] whereas there is a 98% identity with human and murine FGF8e and FGF8f [4]. Human FGF-8a, a monomeric protein has a molecular weight of 21.3kDa with 182 amino acid (aa) residues covering the signal sequence domain, N-terminal domain, FGF domain and proline-rich C terminal sequence [5]. Its three-dimensional structure is composed of two antiparallel beta-sheet and several alpha-helices. The protein contains a conserved heparin-binding domain that is essential for its biological activity. This domain binds to heparin sulfate proteoglycans on the cell surface and helps to localize FGF8a to its target cells.
FGF-8, including the spliced forms, functions by binding the FGF receptors (FGFR) to activate the Ras/MAPK signaling pathway, a key pathway that contributes to several cellular processes. In general, the FGF family is involved in broad cellular and biological processes including cell proliferation, differentiation, survival, and apoptosis [6-8].
Functionally, FGF-8a has been shown to play a major role during prenatal development. It is widely expressed during embryogenesis and is a key player in epithelial-mesenchymal transitions [9]. During gastrulation, it contributes to the organization and induction role and regulates the patterning of organs in the embryos. These organs include the brain, eye, ear, limb, and heart [10-12].
Although, FGF-8 isoforms work in a coordinated and concerted manner, findings have suggested that they also have distinct key roles. FGF-8a is required for morphogenesis and neurogenesis [13]. A study using transgenic mice showed FGF-8a expands the midbrain while FGF-8b showed a transformational activity by transforming the midbrain into the cerebellum [10].
Furthermore, FGF-8a has been used to generate embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)-derived ventral midbrain dopaminergic (DA) neurons [14]. FGF-8a has also been shown to play a role in neuronal induction during neural development and neurite outgrowth from spiral ganglion neurons in vitro [15-16].
Whilst there is limited expression of FGF-8 and its isoforms in the normal adult, increasing studies have shown the presence of FGF-8 in adult tissues and cells including the reproductive tract, peripheral leukocytes, and hematopoietic cells [17-19]. Further functional studies are required to fully delineate the role of FGF-8 and its isoforms in the normal adult.
Customer & collaborator data
Differentiation of induced pluripotent stem cells (iPSCs) into dopaminergic neurons
Qkine animal origin-free growth factors BDNF (Qk050), FGF8a (Qk059), GDNF (Qk051) and TGFb3 (Qk054) can supported the differentiation of iPSCs into mature dopaminergic neurons within 35 days.
Day 35 differentiated mature dopaminergic neurons (A, scale bar = 750 µM, B, scale bar = 300 µM).

Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.

Receive an Amazon gift voucher when you leave us a review.
£25, $30 or €30 for reviews with an image and £10, $15 or €15 for reviews without an image