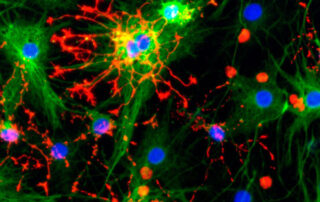
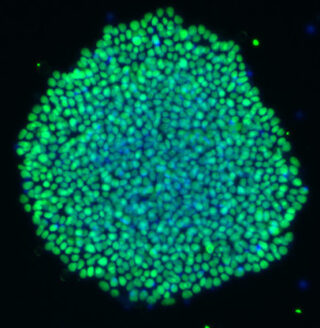
Qkine collaboration with the Bertero Lab, Heart Engineering and Developmental Genomics et al. (HEDGe), University of Turin, Italy
This recent publication in collaboration with Qkine focusses on the optimization of home-brew media for the maintenance of human induced pluripotent stem cells (hiPSCs), using Qkine growth factors to allow cost-effective, practical, and reproducible culture of hiPSCs. Qkine thermostable tag-free FGF2-G3 proteins were found to be highly bioactive, necessitating an 8-fold reduction in concentration for the short (145 aa) form of FGF2-G3 in the optimized media.