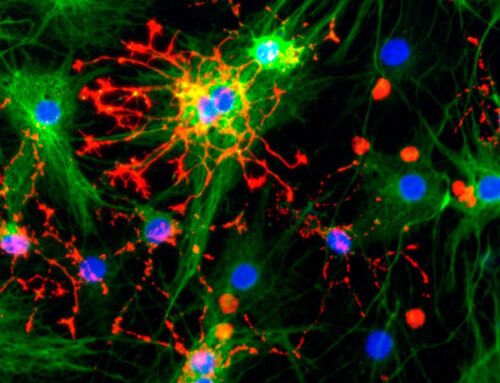
Nurturing Complexity: The role of vascularization in Organoid Advancements
Organoids are exciting models that are rapidly becoming a hopeful platform for many medical advances such as drug screening and disease modeling. As miniature versions of organs, organoids closely mimic the architecture, cellular composition, and functions of their full-sized counterparts. These 3D cell cultures are derived from pluripotent stem cells (PSCs), adult stem cells, or tissue-specific cells and are encouraged to self-organize into structures that replicate the complexity of actual organs. Organoids encapsulate intricate cellular interactions, tissue-specific functions, and some disease behaviors, making them invaluable tools for studying human physiology and pathology in a controlled environment.
However, both PSCs and adult cell-derived organoids have limitations due to the lack of vascularization: the establishment of a comprehensive network of blood vessels to supply nutrients, oxygen, and regulatory growth factors to cells within the organoid.
In human bodies, organs receive essential nutrients and oxygen through vascular networks, essential for the survival and functionality of all organs. Organoids lack such central circulation systems and rely on molecular diffusion for nutritional delivery. The diffusion range is limited without perfusable vessels, restricting the maximum size of organoids in vitro. Without vascularization, nutrients and oxygen can only diffuse a limited distance within a few hundred microns of capillaries into the tissue, leading to a lack of proper nutrient supply and waste removal. This limitation results in poor cell viability, limited growth, and an inability to recapitulate complex physiological processes that require intricate signaling between cells. For organoids to truly mimic the behavior of real organs, a vascular network is indispensable.
Overview of several conducted practical methods for successful organoid vascularization
Like all organoids, brain organoids appear to also have a significant obstacle where oxygen and nutrients cannot be delivered to the innermost parts of the organoid due to the brain organoids lacking vascularization. To address this, a number of practical methods have been conducted. An overview of just a handful of methodologies, is explained below:
1. Vascularizing organoids through co-culture with endothelial cells
The vascularization of organoids, a critical aspect of creating physiologically relevant miniature organs, often involves the co-culture with endothelial cells. Endothelial cells are derived from mesoderm and are required for the formation of new blood vessels.
This interaction mirrors the intricate interaction that occurs during embryonic development and early organogenesis, encouraging endothelial cells to self-assemble into capillary-like structures within the organoid.
In the context of human brain organoids, co-culturing with vascular cells, specifically a monolayer of endothelial cells, has been employed to achieve organoid vascularization. In 2018, Pham et al., successfully vascularized 34-day-old human brain organoids by embedding them in Matrigel, enriched with endothelial cells derived from human iPSCs, along with bone morphogenetic protein 4 (BMP4), vascular endothelial growth factor A (VEGF 165), and fibroblast growth factor 2 (FGF-2). After 34 days of co-culture, these brain organoids displayed a network of penetrating vessels expressing platelet endothelial cell adhesion molecule, a key endothelial marker.
Perhaps the most common method for organoid vascularization involves co-culturing human organoids with human umbilical vascular endothelial cells (HUVECs). In brain organoids vascularized through HUVEC co-culture for over 200 days, Shi et al., found that vascularization initially manifests in the ventricular zone (VZ)-like region, which houses neural stem and progenitor cells (NSPCs), mirroring the developmental patterns of the human brain vasculature. Vascularized brain organoids exhibit more robust growth compared to their non-vascularized counterparts, suggesting that vascularization promotes the proliferation of NSPCs and/or the survival of neurons, even in an in vitro environment.
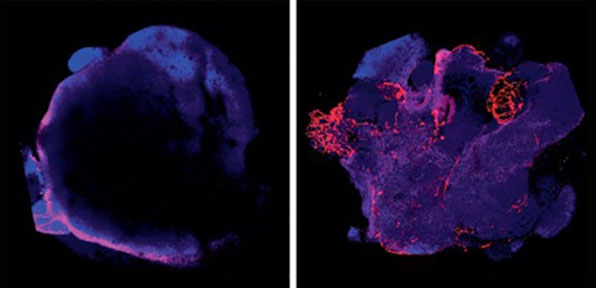
Co-culture of organoids with stem cells differentiated toward an endothelial fate (right), resulting in cortical organoids with a vasculature (red). The control organoids (left) express few endothelial markers. Cakir et al., Nature Methods. Engineering of human brain organoids with a functional vascular-like system.
Related reading
PLoS Biol. 2020
Vascularized human cortical organoids (vOrganoids) model cortical development in vivo.
Authors: Shi Y, Sun L, Wang M, et al.
Neuroreport. 2018
Generation of human vascularized brain organoids.
Authors: Pham MT, Pollock KM, Rose MD, et al.
2. Three-dimensional bioprinting to achieve organoid vascularization
Bioprinting technologies allow researchers to precisely position cells and biomaterials to create intricate structures, including vascular networks. By using bioinks composed of endothelial cells and supportive matrix materials, researchers can fabricate organoids with predefined vascular patterns. Bioprinting allows for fine control over the architecture of the vascular network. It can create highly customized organoids with intricate vasculature.
Bioprinting technologies allow researchers to precisely position cells and biomaterials to create intricate structures, including vascular networks. By using bioinks composed of endothelial cells and supportive matrix materials, researchers can fabricate organoids with predefined vascular patterns. Bioprinting allows for fine control over the architecture of the vascular network. It can create highly customized organoids with intricate vasculature.
Bioprinting has emerged as a transformative technology for creating large-scale tissue constructs with intricate vascular networks. Two primary methods, Filament Deposition Fabrication (FFF) and Fused Deposition Modeling (FDM), are frequently employed to achieve this goal. These techniques involve depositing cell-laden hydrogel filaments in a controlled manner to form specific patterns, offering immense potential for tissue engineering.
Although bioprinting enables the creation of tissues with spatially organized cells and extracellular matrices, it faces challenges related to resolution. The printing resolution is limited, particularly in extrusion-based bioprinting, which has a resolution of about 100 µm, whereas the diameter of typical capillaries is approximately 10 µm.
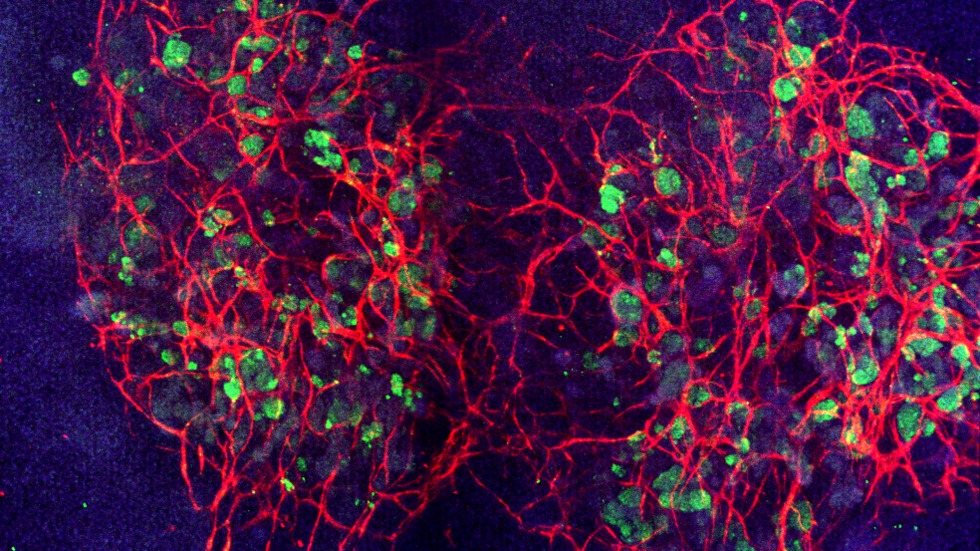
When grown under fluidic shear stress, a network of blood vessels (red) develops to connect kidney cells (green) within and between organoids. Credit: Wyss Institute at Harvard University.
Related reading
Small 16, 1905505. 2020
Bioprinting of Multiscaled Hepatic Lobules within a Highly Vascularized Construct.
Authors: Kang, D. et al.
Biomolecules 11, 966. 2021
Engineering the Vasculature of Stem-Cell-Derived Liver Organoids.
Authors: Zhang, X., Tang, L. & Yi, Q
3. Advancing organoids-on-a-chip for organoid vascularization
Organoids-on-a-chip is a promising solution to the lack of complexity of living environments of 2D culture. This innovative technique involves the construction of organoids within microfluidic devices, with meticulous consideration of the anatomical structure and physiological characteristics of the target organ. While traditional microfluidic organoids lack blood circulation, vascularized organoids-on-a-chip devices offer several advantages for studying brain-related events.
To create organoids-on-a-chip, researchers aim to replicate the 3D mechanical and biochemical environment of the target organ. This process involves leveraging the growth characteristics of cells and utilizing micro-processing technologies, such as soft lithography, to construct the necessary model for cell growth. These models are designed to closely resemble the structure and function of the organ they mimic for disease modeling, insights into pathological processes, pharmacology modeling and aiding drug testing and evaluation.
When applied to brain organoids, vascularized organoid-on-a-chip devices provide several unique benefits. The devices simplify the evaluation of blood-brain barrier (BBB) permeability as well as detailed analyses of BBB function, including calcium imaging to monitor neuronal activity after drug delivery.
Related reading
Stem Cells, 39, 1017–1024 (2021).
Vascularization of Human Brain Organoids.
Authors: Matsui, T. K., Tsuru, Y., Hasegawa, K. & Kuwako, K.
Frontiers in Bioengineering and Biotechnology, 9, (2021).
Review on the Vascularization of Organoids and Organoids-on-a-Chip.
Authors: Zhao, X. et al.
The vascularization of organoids represents a groundbreaking frontier in regenerative medicine and tissue engineering. As techniques continue to advance, organoids hold the potential to revolutionize drug testing, disease research, and transplantation. The integration of vascular networks within organoids brings us closer to realizing their full potential in reshaping the landscape of medical research and treatment.
More resources
Organoids culture
If you are working on organoid cultures, visit our organoid landing page to buy animal-free recombinant growth factors for your cultures. Using animal-free growth factors for organoids can greatly improve the reporducibility of your cultures.
Kidney organoids
Learn how the collaborative project created vascularized kidney organoids and how they advance the field of tissue engineering.
Credit: Wyss Institute at Harvard University.