Currency
Recombinant human/rat/bovine/porcine FGF-10 protein (Qk003)
Human/rat/porcine/bovine FGF10 protein promotes lung organoid formation and induces branching morphology. FGF10 protein is used widely in organoid culture, embryonic stem cell (ESC) and induced-pluripotent stem cell (iPSC) differentiation, and for the study of epithelial to mesenchymal transition and tumor metastasis.
High purity and bioactivity 17 kDa, bioactive domain of human fibroblast growth factor 10, animal-free (AOF) and carrier-protein free (CF).
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
1000µg will be despatched as 2 x 500µg
Buy online with secure credit card or purchase order or email orders@qkine.com
Bulk and stock reservation available
Summary
High purity human FGF10 protein (residues 64-208, Uniprot: O15520)
>98%, by SDS-PAGE quantitative densitometry
17 kDa
Expressed in E. coli
Animal-free (AOF) and carrier protein-free
Manufactured in our Cambridge, UK laboratories
Lyophilized from HEPES/NaCl/mannitol
Resuspend in water at >100 µg/ml, prepare single use aliquots, add carrier protein if desired and store frozen at -20oC or -80oC
Featured applications
Differentiation of embryonic stem cells into gut-like structures, cardiomyocytes and hepatocytes
Epithelial to mesenchymal transition
- Itoh, N. & Ohta, H. Fgf10: A Paracrine-Signaling Molecule in Development, Disease, and Regenerative Medicine. Curr. Mol. Med. 14, 504–509 (2014).
- Zhang, X. et al. Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J. Biol. Chem. 281, 15694–700 (2006).
- Igarashi, M., Finch, P. W. & Aaronson, S. A. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7). J. Biol. Chem. 273, 13230–5 (1998).
- Beer, H.-D. et al. The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: implications for epithelial repair. Oncogene 24, 5269–77 (2005).
- Ohuchi, H. et al. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development 124, 2235–44 (1997).
- Min, H. et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, 3156–61 (1998).
- Zhang, R.-R. et al. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Reports 10, 780–793 (2018).
- McCracken, K. W. et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404 (2014).
human, rat, bovine, porcine
species similarity:
mouse – 94%
frequently used together
Bioactivity
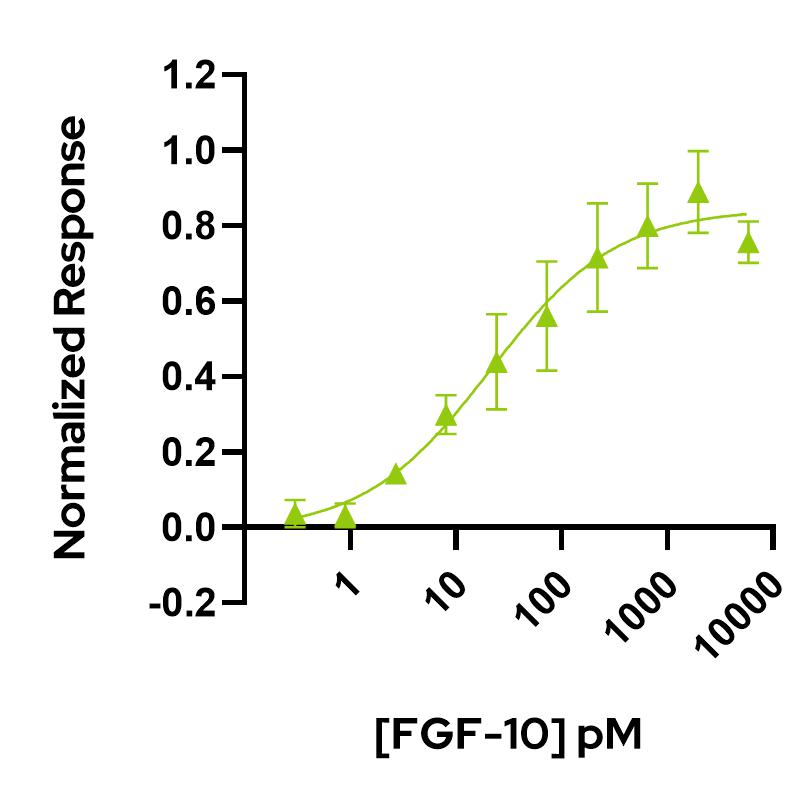
FGF-10 activity is determined using the firefly luciferase reporter assay in stably transfected HEK293T cells. Cells are treated in triplicate with a serial dilution of FGF-10. Firefly luciferase activity is measured and normalized. EC50 = 21.1 pM (0.36 ng/mL).Data from Qk003 lot #104403.
Purity
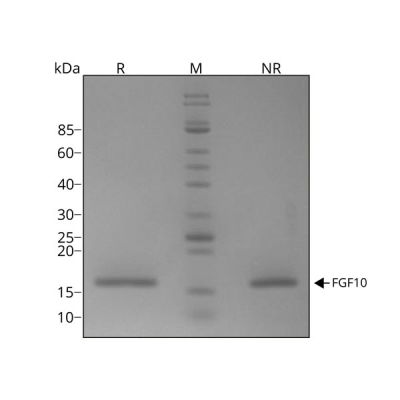
FGF10 migrates as a single band at 17 kDa in non-reducing (NR) conditions and upon reduction (R). No contaminating protein bands are visible.
Purified recombinant human FGF10 protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250. Data from Qk003 lot #010
Further quality assays
Mass spectrometry: single species with expected mass
Endotoxin: <0.005 EU/μg protein (below level of detection)
Recovery from stock vial: >95%
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
protein background
Fibroblast growth factor 10 (FGF10) is involved in a number of different embryo and adult cell and tissue types, including mesenchymal, neuronal and epithelial cells. Human FGF10 protein is expressed in the mesenchyme and functions through interacting with the epithelial FGF Receptor 2b (Fgfr2b)1. It has also been shown to interact weakly with FGF Receptor 1b2. The mature form of human FGF10 protein is an approximately 20 kDa protein highly similar to FGF7 and with a serine-rich region near its N-terminus3. It is secreted by mesenchymal cells and is bound and activated by extracellular FGF-BP4.
Human fibroblast growth factor 10 is first active in the limb bud mesoderm where it creates and maintains FGF signalling with epithelial FGF8, then drives a positive feedback loop accumulating mesenchyme in the growing bud, and finally induces the apical ectodermal ridge which ultimately gives rise to feet and hands5. Lung development is based on the same epithelial-mesenchymal FGF mediations involving FGF10 from the foregut mesenchyme signalling to FGFR2 in the foregut epithelium6.
Furthermore, FGF10 protein is involved in the development of white adipose tissue, heart, liver, brain, kidney, thymus, inner ear, tongue, trachea, eye, prostate, salivary gland and mammary gland. It has been shown to induce migration and invasion of pancreatic cancer cells and to be associated with breast cancer risk, and patients with FGF10 haploinsufficiency present symptoms of chronic obstructive pulmonary disease. Human recombinant FGF10 protein also drives the differentiation of embryonic stem cells into gut-like structures, cardiomyocytes and hepatocytes1.
Customer & collaborator data
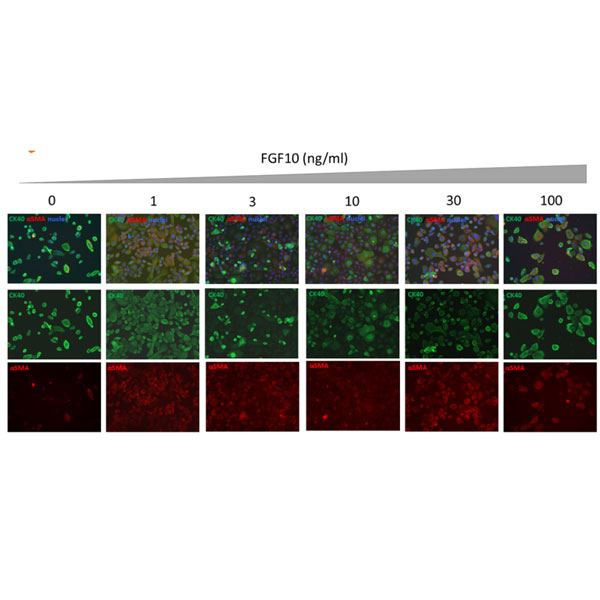
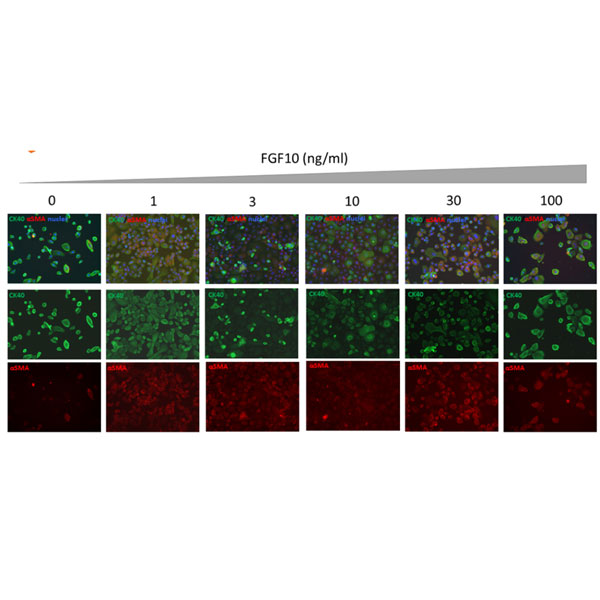
FGF10 supports epithelial to mesenchymal transition (EMT) in human primary keratinocytes. Data and evaluation by Stemnovate Ltd.
Epithelial to mesenchymal transition (EMT) is a crucial morphogenetic process during development in which cells lose their epithelial characteristics and acquire migratory mesenchymal properties. Human FGF10 protein has an important role both during the embryonic EMT (type I) and on cancer cell initiation of metastasis (type III EMT).
Induction of EMT in human primary keratinocytes following treatment with hFGF10. Induction of EMT was evaluated using immunofluorescence staining to determine expression of the epithelial marker (Cytokeratin 14 [CK14]) and mesenchymal marker (α-Smooth Muscle Actin [αSMA]) in human primary epidermal keratinocytes after 4 days treatment with Qk003 hFGF10 (0-100 ng/ml).
FGF10 supports proliferation and promotes epithelial to mesenchymal transition in human primary keratinocytes. Data and evaluation by Stemnovate Ltd.
Epithelial to mesenchymal transition (EMT) is a crucial morphogenetic process during development in which cells lose their epithelial characteristics and acquire migratory mesenchymal properties. FGF10 has an important role both during the embryonic EMT (type I) and on cancer cell initiation of metastasis (type III EMT).
Induction of EMT in primary keratinocytes
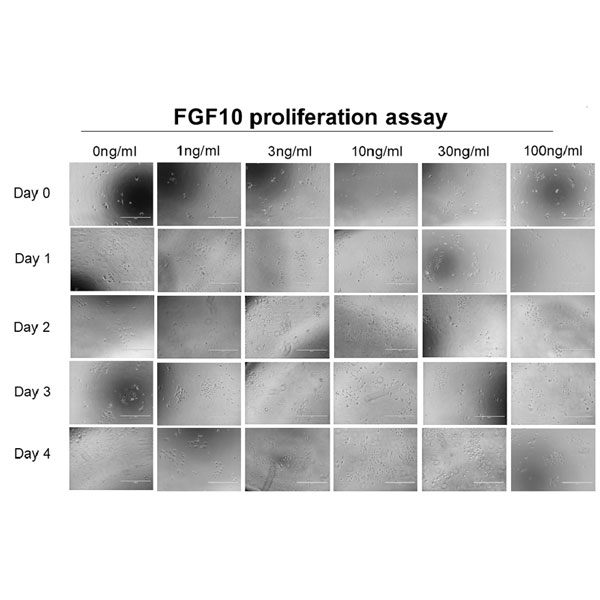
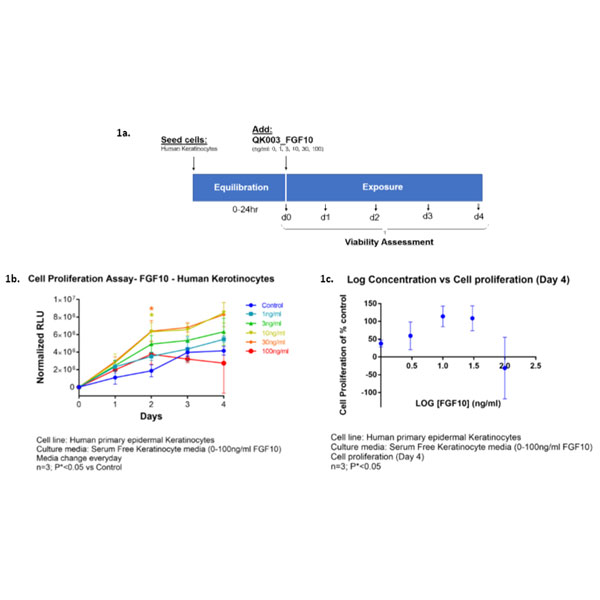
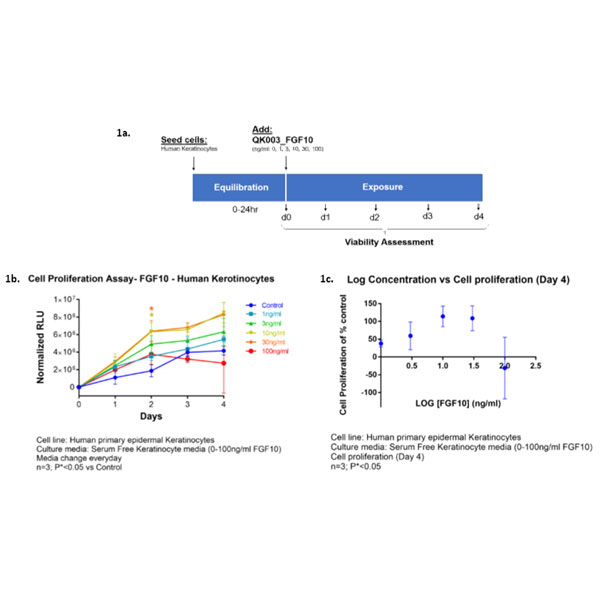
Cell proliferation assays to assess the effect of Qkine FGF10 (0-100 ng/ml) on human primary epidermal keratinocytes in serum-free keratinocyte media. Cells were evaluated at culture days: 0 (baseline), 1, 2, 3, 4 days, as summarized schematically in Figure 1a. Figure 1b shows cell proliferation (Relative Luminescence Unit [RFU]) for days 1, 2, 3, 4 and normalized to day 0 readouts (n=3; P*<0.05 vs control). The log concentration plot in Figure 1c shows percent cell proliferation normalized over untreated control (%) and to day 0 (baseline) after 4 days treatment (n=3; P*<0.05). The maximal cell proliferation was observed at ~10ng/ml FGF10 and a reduction in cell number/viability as observed at 100 ng/ml. Data provided by Stemnovate Ltd, Cambridge, UK.
Gastic organoids – Dylan Liabeuf
Hu-FGF10 allowed us to derive and maintain patient-derived gastric organoids as part of our cocktail. Thanks to the high purity of their cytokines and quality-price ratio overcome by far our usual providers.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.




What others are saying
There are no contributions yet.