Introduction
Since their introduction in 2006, induced pluripotent stem cells (iPSCs) have revolutionized research and therapeutic development. iPSCs are invaluable for disease modeling due to their unique ability to differentiate into nearly any cell type, making them a powerful tool for studying genetic disorders, infections, and cancers. Their role in high-throughput drug screening and toxicity studies has significantly advanced our understanding of disease mechanisms across various conditions and enabling the customization of treatment strategies based on individual genetic profiles [1]. Additionally, iPSCs offer an ethical and sustainable alternative to embryonic stem cells, further broadening their application in research and regenerative medicine.
One key application of iPSCs is hepatocellular differentiation, a critical area within regenerative medicine. Hepatocyte-like cells (HLCs) derived from iPSCs exhibit many functions of primary liver cells. This advancement holds promise for liver disease research and the development of personalized treatment pathways. These advancements may ease the demand for liver transplants by providing an alternative cell source for therapeutic use [2]. Furthermore, HLCs are powerful models for studying liver metabolism, genetic liver diseases, and drug interactions, which are vital for evaluating drug safety and liver-specific toxicities before clinical trials.
Producing functional hepatocytes from iPSCs requires precise activation of key signaling pathways, such as Wnt, activin/nodal, FGF, and BMP, to replicate the stages of liver development. The sequential activation of these pathways supports early liver cell differentiation, while maturation is driven by factors such as hepatocyte growth factor (HGF) and oncostatin M (OSM) [2,3]. This precise mimicry of liver development is essential for achieving fully functional HLCs, enhancing their utility in studying liver disease progression, improving drug screening, and refining gene-editing techniques for therapeutic applications.
The advancements in iPSC-derived hepatocyte differentiation underscore the potential for combining cutting-edge stem cell technologies with molecular biology to transform research, drug discovery, and regenerative therapies for liver-related conditions. This application note defines a protocol and key considerations for generating HLCs, highlighting the role of specific growth factors in achieving optimal differentiation outcomes.
Materials and methods
Cell culture and maintenance
iPSCs were passaged twice weekly using 0.5 mM EDTA for detachment and seeded onto vitronectin (Qk120, 5 µg/ml) coated 6-well plates at a 1:6 split ratio. The cells were cultured in E8-like media, with spent media replaced the day after passage with 5 ml of fresh E8-like media, allowing the cells to follow a weekend-free media change routine. For further details on this process, please refer to our guide on Weekend-free human induced pluripotent stem cell culture, which highlights the use of thermostable FGF-2 (bFGF) from Qkine, together with our animal origin-free TGF-β1 and vitronectin, for enhanced colony homogeneity.
iPSC differentiation into hepatocyte-like cells
The HLC differentiation workflow schematic (Figure 1) outlines the steps involved in differentiating iPSCs into hepatocyte-like cells and assessing differentiation through the expression of hepatocyte specific markers.
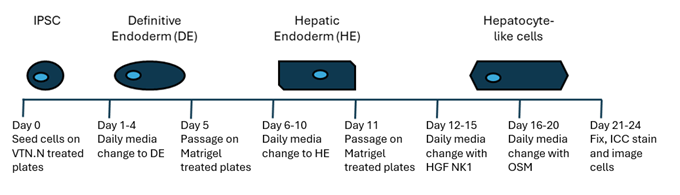
Figure 1. Schedule for HLC differentiation and evaluation.
Day 0
iPSCs were detached using AccutaseTM and seeded at 150,000 cells/well onto vitronectin (Qk120) (5 µg/ml) coated 6-well plates in E8-like media supplemented with ROCK inhibitor (Y-27632, 10 µM). The following day (Day 1) and daily until Day 5, the cells were fed with Endoderm medium (Table 1).
| Component | Final Concentration |
|---|---|
| RPMI media | 1x |
| B-27 (50x) | 1x |
| Recombinant activin A (Qk001) | 100 ng/ml |
| Recombinant BMP-4 (Qk038) | 10 ng/ml |
| Recombinant FGF2-G3 (Qk053) | 40 ng/ml |
| CHIR99021 | 3 µM |
| Ly294002 | 10 µM |
Table 1. Endoderm medium. Components added aseptically prior to use.
Day 5
Endoderm cells were detached using AccutaseTM and seeded at 150,000 cells/well onto Matrigel (0.25 µg/ml) coated 6-well plates in Hepatocellular Endoderm medium supplemented with ROCK inhibitor (Y-27632, 10 µM). The following day (Day 6) and daily until Day 10, the cells were fed with Hepatocellular Endoderm medium (Table 2).
| Component | Final Concentration |
|---|---|
| RPMI media | 1x |
| B-27 (50x) | 1x |
| Non-essential amino acids | 1x |
| Recombinant BMP-4 (Qk038) | 20 ng/ml |
| Recombinant FGF2-G3 (Qk053) | 10 ng/ml |
Table 2. Hepatocellular Endoderm medium. Components added aseptically prior to use.
Day 11
Hepatocellular endoderm cells were detached using AccutaseTM, collected in Hepatozyme Differentiation medium (Table 3) and seeded at 500,000 cells/well onto Matrigel (0.25 µg/ml) coated 6-well plates in Hepatic Progenitor Differentiation medium supplemented with ROCK inhibitor (Y-27632, 10 µM) and HGF (Table 4). The following day (Day 12) and daily until Day 15, cells were fed with Hepatozyme Differentiation Medium with HGF NK1 (Table 4).
| Component | Final Concentration |
|---|---|
| Hepatozyme media | 1x |
| B-27 (50x) | 1x |
| Non-essential amino acids (100x) | 1x |
Table 3. Hepatozyme Differentiation medium. Components added aseptically prior to use.
| Component | Final Concentration |
|---|---|
| Hepatozyme media | 1x |
| B-27 (50x) | 1x |
| Non-essential amino acids | 1x |
| Recombinant HGF NK1 (Qk013) | 20 µg/ml |
Table 4. Hepatozyme Progenitor Differentiation medium. Components added aseptically prior to use.
Day 16
From Day 16 until Day 20, hepatic progenitor cells were medium changed daily with 1 ml Hepatocellular Differentiation medium supplemented with 10 µg/ml OSM (Table 5).
| Component | Final Concentration |
|---|---|
| Hepatozyme media | 1x |
| B-27 (50x) | 1x |
| Non-essential amino acids (100x) | 1x |
| Recombinant OSM (Qk049) | 10 µg/ml |
Table 5. Hepatocellular Differentiation medium with OSM. Components added aseptically prior to use.
Immunocytochemistry
On Day 21, cells were fixed with 4% Paraformaldehyde, then blocked and permeabilized with 10% donkey serum diluted in 0.1% Triton X-100. Specific antibodies for hepatocyte markers – Anti-Albumin antibody, Anti-alpha 1 Antitrypsin antibody, or Anti-alpha 1 Fetoprotein – were applied for immunostaining overnight at 4 °C. Cells were then washed and incubated with secondary antibodies Goat anti-Rabbit IgG H&L AlexaFluorTM 488 and Hoechst 33258, followed by imaging in phosphate buffered saline.
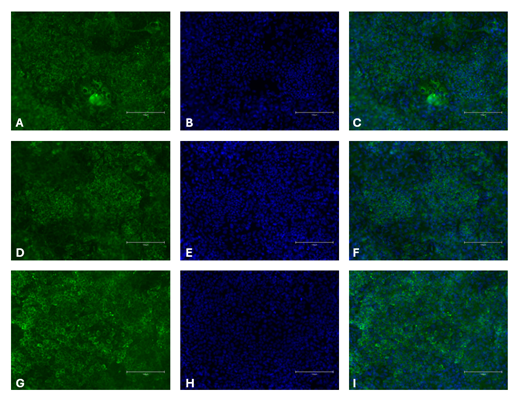
Figure 2. Immunocytochemistry of hepatocellular markers in differentiated iPSCs. Albumin [green, A], Hoechst 33258 [blue, B], combined albumin and Hoechst [C], Alpha-1-fetoprotein [green, D], Hoechst 33258 [blue, E], combined alpha-1-fetoprotein and Hoechst [F], and alpha 1 antitrypsin [green, G], Hoechst 33258 [blue, H], alpha 1 antitrypsin and Hoechst [I]. Images were acquired using the EVOS M5000 system (scale bar = 150 µm).
Results and conclusion
The growing significance of iPSCs in clinical and research applications stems from their unique ability to differentiate into all somatic cell types in the human body. Successful differentiation of iPSCs into desired cell types, such as hepatocyte-like cells, relies on maintaining pluripotency during culture and achieving homogenous differentiation. The use of optimized media, containing highly pure and bioactive growth factors, is critical for reproducibility achieving for driving efficient, high-quality differentiation.
The data presented in this application note demonstrate that high purity growth factors from Qkine, including activin A (Qk001), FGF2-G3 (Qk053), BMP-4 (Qk038), HGF NK1 (Qk013) and OSM (Qk049), support the differentiation of iPSCs into hepatocyte-like cells. These growth factors not only ensure robust and reliable differentiation but also reduce variability, a key requirement for both research and therapeutic applications.
This work highlights the importance of high-quality reagents in overcoming challenges associated with iPSC differentiation, such as inconsistent cell performance or variability in outcomes. By using the broad portfolio of animal origin-free growth factors from Qkine, researchers can enhance the reproducibility of long-term, complex cell cultures and advance applications in regenerative medicine, drug discovery, and disease modeling.
Further information
Qkine growth factors are manufactured to the highest quality standards and are free from animal-derived contaminants, delivering low endotoxicity and high purity. At Qkine, we are committed to raising the standards of growth factors, cytokines and related proteins to better support long-term and complex cell cultures. We are a science-led team, please reach out with any questions or requests to support@qkine.com.