Currency
Recombinant human FGF-4 protein (Qk004)
Human FGF-4 protein is used for the proliferation and differentiation of embryonic and induced-pluripotent and tissue (mesenchymal) stem cells and promotes neural stem cell proliferation. FGF-4 is an important component of cardiac, intestinal and other organoid culture media.
High purity and bioactivity 14 kDa, bioactive domain of human fibroblast growth factor 4, animal origin free (AOF) and carrier-protein free.
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
1000µg will be despatched as 2 x 500µg
Buy online with secure credit card or purchase order or email orders@qkine.com
Bulk and stock reservation available
Summary
High purity human FGF4 protein (residues 79-206, Uniprot: P08620)
>98%, by SDS-PAGE quantitative densitometry
14 kDa
Expressed in E. coli
Animal-free (AOF) and carrier protein-free
Manufactured in our Cambridge, UK laboratories
Lyophilized from HEPES/NaCl/mannitol
Resuspend in water at >100 µg/ml, prepare single use aliquots, add carrier protein if desired and store frozen at -20°C or -80°C
Featured applications
Hindgut organoids from iPSC-derived human posterior gut endoderm cells7
Neural stem cell proliferation and neuronal differentiation
- Beenken, A. & Mohammadi, M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–53 (2009).
- Coutu, D. L. & Galipeau, J. Roles of FGF signaling in stem cell self-renewal, senescence and aging. Aging (Albany. NY). 3, 920–33 (2011).
- Kosaka, N., Sakamoto, H., Terada, M. & Ochiya, T. Pleiotropic function of FGF-4: Its role in development and stem cells. Dev. Dyn. 238, 265–276 (2009).
- Kosaka, N. et al. FGF-4 regulates neural progenitor cell proliferation and neuronal differentiation. FASEB J. 20, 1484–1485 (2006).
- Farré, J. et al. FGF-4 increases in vitro expansion rate of human adult bone marrow-derived mesenchymal stem cells. Growth Factors 25, 71–76 (2007).
- Mayshar, Y. et al. Fibroblast Growth Factor 4 and Its Novel Splice Isoform Have Opposing Effects on the Maintenance of Human Embryonic Stem Cell Self-Renewal. Stem Cells 26, 767–774 (2008).
- Zhang, R.-R. et al. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Reports 10, 780–793 (2018).
- McCracken, K. W. et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404 (2014).
human
species similarity:
mouse – 91%
porcine – 91%
bovine – 90%
rat – 80%
Frequently used together
Bioactivity
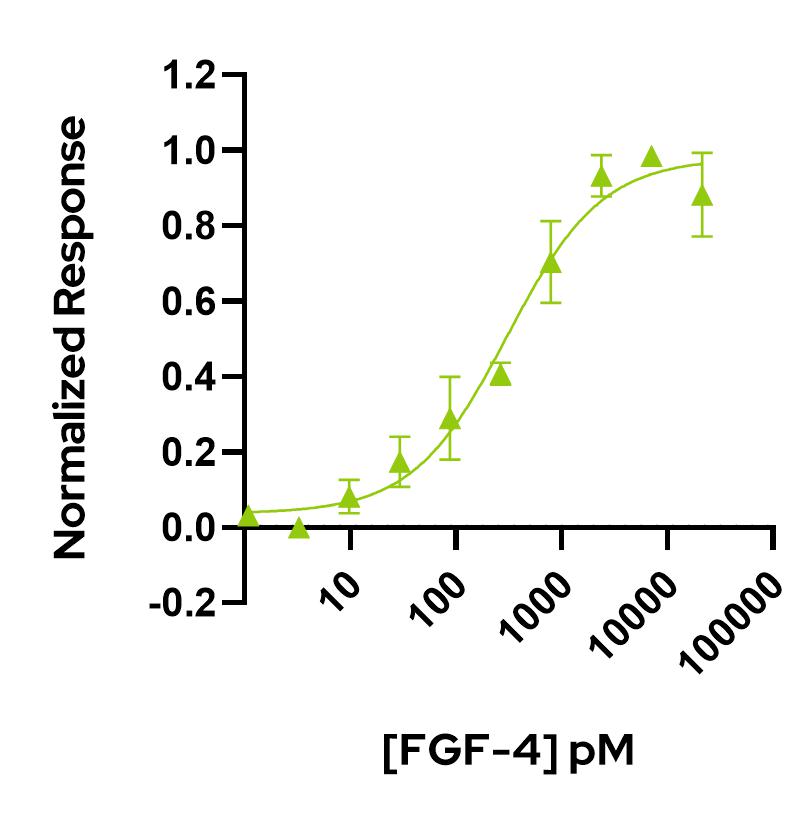
FGF-4 activity is determined using the firefly luciferase reporter assay in stably transfected HEK293T cells. Cells are treated in triplicate with a serial dilution of FGF-4. Firefly luciferase activity is measured and normalized.
EC50 = 306.5 pM (4.3 ng/mL).
Data from Qk004 lot #104330.
Purity
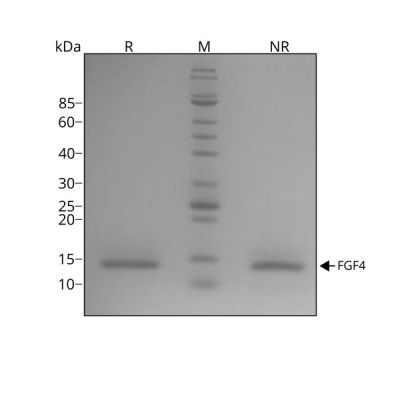
Recombinant human FGF4 protein migrates as a single band at 14 kDa in non-reducing conditions (NR) and upon reduction (R). No contaminating protein bands are visible.
Purified recombinant protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250.
Data from Qk004 lot #010
Further quality assays
Mass spectrometry: single species with expected mass
Endotoxin: <0.005 EU/μg protein (below level of detection)
Recovery from stock vial: >95%
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
Fibroblast growth factor 4 (FGF4) is a member of the FGF superfamily with a physiological role in the regulation of proliferation and differentiation in embryonic stem cells and tissue stem cells1-3.
Recombinant human FGF4 protein promotes neural stem cell proliferation and neuronal differentiation in the postnatal brain4, increases the proliferation rate of human adult bone-marrow derived mesenchymal stem cells5 and supports the maintenance, proliferation and self-renewal properties of human embryonic stem cells6.
Synergism between FGF4 and WNT signalling acts to form hindgut organoids from iPSC-derived human posterior gut endoderm cells7 and recombinant human FGF4 protein is used to mimic embryonic intestinal development during directed differentiation in culture of pluripotent stem cells into intestinal organoids.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.



What others are saying
There are no contributions yet.