The ability to generate dopaminergic neurons from iPSCs holds immense potential for both research and therapy. iPSC-derived dopaminergic neurons provide a valuable model for studying the mechanisms underlying neurodegenerative diseases such as Parkinson’s disease and the dysregulation of dopaminergic signaling in psychiatric disorders like schizophrenia. As research progresses, the translation of these findings into clinical applications holds the promise of transformative impacts on patient care and disease management.
Differentiation of induced pluripotent stem cells (iPSCs) into dopaminergic neurons requires specific conditions to obtain the desired cell type. This application note will describe the method we use to differentiate human iPSCs into functional dopaminergic neurons, using a variety of media types that utilize a series of Qkine growth factors and cytokine supplements at different stages of key differentiation steps.
Introduction
Dopaminergic neurons are specialized nerve cells responsible for the synthesis, storage, and release of the neurotransmitter dopamine. These neurons play a pivotal role in several critical brain functions, including motor control, reward, motivation, and regulation of mood [1]. Dopaminergic neurons are primarily located in the substantia nigra and the ventral tegmental area of the brain. They project to various brain regions, including the striatum, prefrontal cortex, and limbic system, forming complex networks that influence a variety of behaviors and physiological processes [2].
Dopaminergic neurons are crucial in motor control, as evidenced by their degeneration in Parkinson’s disease, a condition characterized by motor symptoms such as tremors, rigidity, and bradykinesia. In psychiatric disorders, dysregulation of dopaminergic signaling is implicated. For instance, hyperactivity of dopaminergic pathways is associated with the positive symptoms of schizophrenia, while diminished dopamine function is linked to anhedonia and lack of motivation seen in depression [2]. Differentiating iPSCs into dopaminergic neurons is an area of research with significant implications for treating neurological disorders, especially Parkinson’s disease.
Materials and Methods
Cell culture and maintenance
Human iPSCs were cultured in a E8-like media in 6-well plates coated with vitronectin Qk120 (5 µg/ml) using a weekend free media changing process. Passages were preformed using 0.5 µM EDTA every 3-4 days and split using a 1:6 ratio to lift cells to ensure high quality iPSCs before starting the differentiation process.
iPSC differentiation to midbrain-specified floor plate progenitor cells
When iPSCs reached the desired confluence and quality, they were passaged using Accutase® and seeded into a Geltrex-coated 24-well plate at a density of 3.8×105 cells / ml. They were then allowed to adhere until achieving 90-100% confluency, forming a monolayer.
Neural induction and dopaminergic specification
iPSCs are first directed to become neural progenitor cells (NPCs) by dual SMAD inhibition, using SB431542, which inhibits pathways that maintain pluripotency and promotes neural differentiation towards the development of dopaminergic neurons using key molecules like fibroblast growth factor 8 (FGF-8), which are critical for the midbrain dopaminergic neuron specification.
Maturation and Maintenance
The final stages involve the maturation of these cells into functional dopaminergic neurons (figure 1), which can be supported by factors such as brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF) and transforming growth factor b3 (TGF-ß3).
Day 1: Media refreshed using Induction Media 1
| Induction Media 1 | |
|---|---|
| 85% | Knockout DMEM |
| 15% | Knockout serum replacement |
| 1x | Glutamax |
| 1x | MEM Non-Essential Amino Acids (NEAA) |
| 10 mM | 2-Mercaptoethanol |
| 100 nM | LDN193189 |
| 10 µM | SB431542 |
Days 2 & 3: Media refreshed using Induction Media 2
| Induction Media 2 | |
|---|---|
| Induction media 1 plus: | |
| 100 nM | SAG hydrochloride |
| 2 µM | Puromorphamine |
| 100 ng/ml | Human FGF-8a (Qk059) |
Days 4 & 5: Media refreshed using Induction Media 3
| Induction Media 3 | |
|---|---|
| Induction media 1 plus: | |
| 100 nM | SAG hydrochloride |
| 2 µM | Puromorphamine |
| 100 ng/ml | Human FGF-8a (Qk059) |
| 3 µM | CHIR99021 |
Days 6 & 7: Media refreshed using Induction Media 4
| Induction Media 4 | |
|---|---|
| 63.2% | Knockout DMEM |
| 11.6% | Knockout serum replacement |
| 24.8% | Neurobasal medium |
| 0.13% | N-2 supplement |
| 0.26% | B-27 Supplement |
| 1x | Glutamax |
| 1x | MEM NEAA |
| 10 µM | 2-Mercaptoethanol |
| 100 nM | LDN193189 |
| 3 µM | CHIR99021 |
| 100 nM | SAG hydrochloride |
| 2 µM | Puromorphamine |
| 100 ng/ml | Human FGF-8a (Qk059) |
Days 8 & 9: Media refreshed using Induction Media 5
| Induction Media 5 | |
|---|---|
| 42.3% | Knockout DMEM |
| 7.6% | Knockout serum replacement |
| 49.3% | Neurobasal medium |
| 0.26% | N-2 supplement |
| 0.5% | B-27 Supplement |
| 1x | Glutamax |
| 1x | MEM NEAA |
| 10 µM | 2-Mercaptoethanol |
| 100 nM | LDN193189 |
| 3 µM | CHIR99021 |
Days 10 & 11: Media refreshed using Induction Media 6
| Induction Media 6 | |
|---|---|
| 21% | Knockout DMEM |
| 3.8% | Knockout serum replacement |
| 74% | Neurobasal medium |
| 0.38% | N-2 supplement |
| 0.74% | B-27 Supplement |
| 1x | Glutamax |
| 1x | MEM NEAA |
| 10 µM | 2-Mercaptoethanol |
| 100 nM | LDN193189 |
| 3 µM | CHIR99021 |

Figure 1. Day 20 maturing midbrain-specified floor plate progenitor cells differentiating into floor plate spheres prior to sphere isolation passage (scale bar = 300 µM)
Day 20: Floor plate sphere isolation passaging
Cells were passaged using a combination of Accutase® and Papain to detach them, then washed and filtered with DMEM/F-12 containing DNase I. Afterward, they were washed and filtered again with DMEM/F-12 alone. The cells were then seeded at a density of 8.645 x 105 cells/ml into a Geltrex-coated 24-well plate containing Seeding Media.
Day 21-30, 32 and 34: Media refreshed using Maturation Media.
As prepared on Day 14-19
Results
At Day 35, mature dopaminergic neurons could be visualized (figure 2).
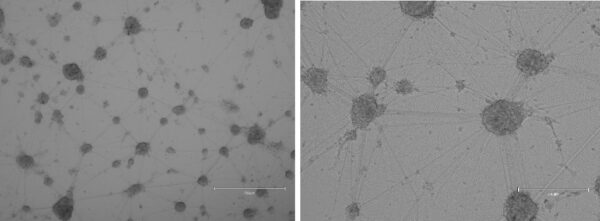
Figure 2. Day 35 differentiated mature dopaminergic neurons (A, scale bar = 750 µM, B, scale bar = 300 µM).
Conclusion
The differentiation of iPSCs into dopaminergic neurons represents a significant tool for advancement in regenerative medicine and neuroscience. The ability to reliably achieve consistent high-quality results can enhance our understanding of the fundamental processes governing neural development and degeneration, and lead toward innovative treatments for neurodegenerative diseases like Parkinson’s.
The data presented in this application note demonstrate that using Qkine animal origin-free growth factors FGF-8a (Qk059), BDNF (Qk050), GDNF (Qk051) and TGF-ß3 (Qk054) can support the differentiation of iPSCs into mature dopaminergic neurons within 35 days.
Further Information
Qkine growth factors are manufactured to the highest of quality standards and are free from animal-derived contaminants, delivering low endotoxicity and high purity. At Qkine, we are committed to raising the standards of growth factors, cytokines and related proteins to better support long-term and complex neural stem cell culture. We are a science-led team, please reach out with any questions or requests to support@qkine.com.
References
[1] O Klein, M. et al. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cellular and Molecular Neurobiology. 2019 Jan;39(1):31-59. doi: 10.1007/s10571-018-0632-3
[2] Zhuang, Y. et al. Mechanism of dopamine binding and allosteric modulation of the human D1 dopamine receptor. Cell Research 2021 May;31(5):593-596. doi.org/10.1038/s41422-021-00482-0
Publications
Preprint. 2023
Profiling human hypothalamic neurons reveals a candidate combination drug therapy for weight loss.
Authors: Chen, H.-J. C. et al.
Labs: John C. Marioni, European Bioinformatics Institute and Florian T. Merkle, University of Cambridge
Featuring Qkine BDNF (Qk050)
Protocols. 2023
Differentiation of RGC Induced Neurons (RGC-iNs).
Authors: Agarwal, D. & Wahlin, K.
Lab: Karl Wahlin, University of California San Diego
Featuring Qkine GDNF (Qk051) and BDNF (Qk050)
Npj Regen. Med. 2023
Human retinal ganglion cell neurons generated by synchronous BMP inhibition and transcription factor mediated reprogramming.
Authors: Agarwal, D. et al.
Lab: Karl Wahlin, University of California San Diego
Featuring Qkine GDNF (Qk051) and BDNF (Qk050)