Pep talk - October 2025

Qkine launches cell therapy grade recombinant proteins to support next-generation therapies
At the start of this month Qkine launched its new Cell Therapy Grade range of proteins with extended QC to be suitable for cell and gene therapy manufacturing.
Cell therapy is rapidly transforming modern medicine, offering new hope for patients with cancer, autoimmune conditions, and genetic disorders. However, the development and manufacturing of these therapies demand reagents of uncompromising quality, consistency, and safety. Qkine’s new Cell Therapy Grade proteins address this critical need by delivering:
- Defined manufacturing standards – Produced under controlled conditions with full traceability and regulatory support documentation
- Enhanced quality attributes – High purity, animal origin-free, low endotoxin levels, and reduced host cell protein/DNA contamination
- Scalability for clinical workflows – Consistent performance from research-grade to therapy-grade materials, enabling seamless transition to GMP production
- Regulatory readiness – Comprehensive Certificates of Analysis (CoA), Safety Data Sheets (SDS), and technical dossiers to support IND and clinical submissions
The portfolio supports a wide range of applications, including CAR-T and TCR therapies, NK cell expansion, iPSC differentiation, and ex vivo gene editing workflows.
In the first phase 14 proteins have been released, but all our current research use portfolio is available for CTG manufacturing on request, contact us for more information customerservice@qkine.com.
For more information on our new cell therapy grade range:
Learn more about our animal origin-free cell therapy grade manufacture

UK Stem Cell Network inaugural event
Qkine supported the re-forming of a UK-wide stem cell network since the idea was raised by the committee over a year ago. Although there are several successful regional stem cell networks in place across the UK, a united network was a clearly valuable and missing initiative. The hope was that a wider network of knowledge sharing across UK stem cell scientists could be created and the inaugural meeting would be a initial taster for what this network could look like and whether their was a desire for it.
However, the hard work of the committee, leveraging of years long connection-building in the stem cell community across academia and biotech resulted in a major event which surpassed all expectations. In the run up to the meeting the goal of the committee was clear, to include scientists from all regions of the UK, of all levels and all stem cell research areas. The event was a triumph, the quality of the science was excellent with a range of topics and presenters from different levels of experience.
As platinum co-sponsors Qkine and Stem Cell Technologies hosted the networking evening at the beautiful Manchester Museum in the Fossils and Living Worlds exhibition. It was an excellent event with photobooth and magician, it had a real party atmosphere and was a wondering opportunity to build connections.
There is no doubt the UK Stem Cell network will continue to support and elevate stem cell research across the UK, by providing a central point of contact and collaboration for researchers and by supporting and promoting local networks.
Visit the UKSCN website for more information.

Our new career blog series
Laura’s role at Qkine may say Office Manager and QMS Assistant, but if you know her, you’ll know she’s so much more than that. She’s the kind of person who thrives on variety, always lends a helping hand, and keeps the engine room of Qkine running with precision, care, and an impressive number of color-coded email flags.
We caught up with her to learn more about her role, what keeps her grounded, and the unexpected path that brought her to biotech.


Why are Qkine’s new products labelled cell therapy grade and not GMP grade?
Qkine have recently released a range of growth factors and cytokines specifically designed to be suitable for cell and gene therapy manufacturing. Most recombinant protein manufacturers would describe these as 'GMP grade' but we haven't, so why are the products labelled cell therapy grade and not GMP grade?
Our latest blog explains what exactly is GMP and how our cell therapy grade proteins comply with GMP and ISO standards.
Animal origin-free protein manufacture for cell therapy
We have previously discussed the issues surrounding animal-derived products for research and why animal origin-free (AOF) alternatives are preferred. These include the potential for cross-contamination with other bioactive proteins or contaminants, which can introduce unwanted variability. When considering proteins used for the development of cell therapy, further assurance of purity and consistency is paramount, particularly due to the potential risk of harm through the application of an improperly manufactured product.
Read our blog explaining the need for animal origin-free proteins for cell therapy.

Did you miss?
Why animal origin-free?
All Qkine proteins are animal origin-free, but why is this so important? And why aren’t all recombinant proteins produced in animal free expression systems?
Qkine shipping charge policy – effective 1st September 2025
From 1st September, Qkine will be introducing updated delivery options designed to give you more choice, flexibility and reward ongoing support.
What's new?
Recombinant human TGF-β1 PLUS™ protein (Qk010-CTG)
Qkine human cell therapy grade TGF-β1 PLUS™ protein is the first entirely animal origin-free recombinant human TGF-β1 protein for increased safety and purity for translational research and cell and gene therapy manufacturing.
Qkine cell therapy grade high purity animal origin-free proteins are manufactured as GMP grade equivalents in an ISO 9001:2015-certified facility, under ISO 20399:2022 standards with GMP compliance, defined quality criteria and documentation.
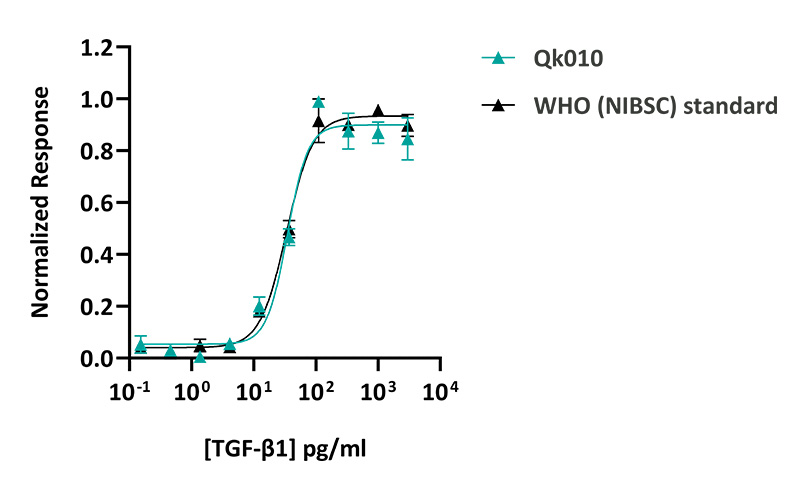
Qkine TGF-β1 PLUS™ was as bioactive as the WHO (NIBSC) standard TGF-β1. TGF-β1 PLUS™ activity was determined using a CAGA luciferase reporter assay in transiently transfected HEK293 cells. Transfected cells were treated in triplicate with a serial dilution of TGF-β1 PLUS™ for 6 hours. Firefly activity was measured and normalized to the control Renilla luciferase activity. Qk010 #204560 EC50 = 35 pg/ml (1.46 pM), WHO (NIBSC) EC50 = 34 pg/ml.

Recombinant human IL-34 PLUS™ protein (Qk091)
Qkine have developed the only commercially available bioactive animal origin-free IL-34!
Qkine have optimized the sequence and manufacture of IL-34 PLUS™ to produce a highly and consistently bioactive protein (patent pending GB 2514413.0). Qk091 has a molecular weight of 25 kDa as a monomer and is supplied as a non-covalent dimer, highly pure, animal origin-free, tag free and carrier free for reproducible microglial differentiation and immune cell culture.
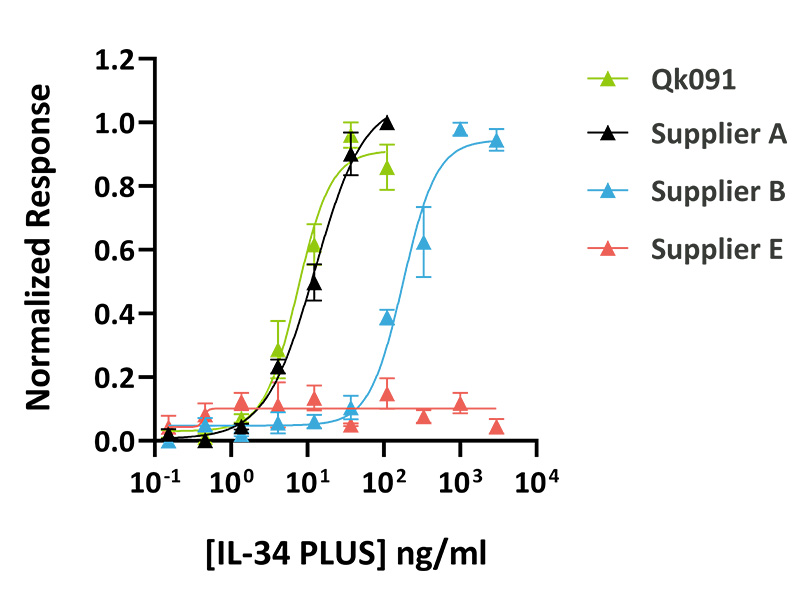
Qkine animal origin-free IL-34 PLUS™ was as bioactive as a mammalian cell-expressed IL-34 from an alternative supplier. IL-34 activity was determined using an SRE reporter assay in HEK293 cells co-transfected with the CSF1R receptor. Transfected HEK293 cells were treated in triplicate with a serial dilution of Qkine IL-34 PLUS™ (Qk091, green), mammalian cell-expressed IL-34 (Supplier A, black) and (Supplier B, blue) or bacterially-expressed IL-34 (Supplier E, pink) for 3 hours. Firefly activity was measured and normalized to the control Renilla luciferase activity.
Application notes
Our cell culture lab is hard at work testing our growth factors and cytokines in complex differentiation protocols. At Qkine data transparency is our promise and we like to help by testing and publishing validated protocols.

Differentiation of induced pluripotent stem cells (iPSCs) into microglia
In this application note, we demonstrate that high purity, animal origin-free growth factors from Qkine - including BMP-4 (Qk038), FGF2-G3 (Qk052), SCF (Qk078), VEGF 165 (Qk048), IL-3 (Qk090), M-CSF (Qk075), Flt3 ligand (Qk087), IL-34 PLUSTM (Qk091) and GM-CSF (Qk076) - support robust and reliable differentiation of iPSCs into microglial cells.
Recent publications
Modeling intestinal epithelial function and polarity using human iPSCs under Air–Liquid interface culture
Tanaka, E et al. Biochemical and Biophysical Research Communications, September 2025
Researchers created a human iPSC-derived Air–Liquid interface culture system which provides a physiologically polarized epithelial model for studying intestinal biology, drug responses, and polarized EV secretion, with potential applications in personalized medicine.
Used Qkine human noggin (Qk034)
Protocol for generation and utilization of patient-derived organoids from multimodal specimen
All our growth factors are manufactured within a stringent quality framework, ensuring high-quality proteins that maintain robust, reproducible, and physiologically relevant stem cell and organoid cultures that adhere to our Nine-point Quality Commitment.
Upcoming Events
- Cambridge Stem Cell Institute Symposium | 13-14 October 2025 | Cambridge, UK
- German Stem Cell Network Conference 2025 | 15-17 October 2025 | Munich, Germany
- EMBL Organoids: Modelling organ development and disease in 3D culture | 22-25 October 2025 | Heidelberg, Germany
- Annual Oxford Stem Cell Institute Symposium | 3-4 November 2025 | Oxford, UK
- Advanced Therapies USA 2025 | 18-19 November 2025 | Philadelphia, USA
- Alternative Protein 4.0 | 21/28 November 2025 | Online Event
- 3rd International Conference of CellAgri Portugal | 27-28 November 2025 | Olhão, Portugal
- ATMP Sweden 2025 | 27-28 November 2025 | Gothenburg, Sweden
We'd always love to meet you at any of events, contact support@qkine.com to make an appointment.
What’s next?
Qkine are committed to producing the most high quality and bioactive growth factors and cytokines, coming soon to our extensive portfolio:
- Recombinant human activin B protein (Qk024) - Expected October 2025
- Recombinant human activin C protein (Qk026) - Expected October 2025
If you wish to be notified when proteins are released, contact us customerservice@qkine.com
