 Recombinant human GDF-5 protein (Qk070)
Recombinant human GDF-5 protein (Qk070)Recombinant human GDF-5 protein (Qk070)
£205.00 – £2,900.00

Growth differentiation factor 5 (GDF-5) plays a crucial role during embryonic development and tissue homeostasis and is specifically involved in the development of the skeletal system. Recombinant human GDF-5 protein is commonly used for the differentiation and maintenance of induced pluripotent stem cells, embryonic stem cells, or bone marrow-derived mesenchymal stem cells into osteoblasts and chondrocytes.
Human GDF-5 is a dimer with a molecular weight of 27 kDa. This protein is animal origin-free, carrier protein-free, and tag- free with exceptional lot-to-lot consistency. GDF-5 is suitable for reproducible and high-quality chondrocytes and osteoblasts.
In stock
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
£205.00 – £2,900.00
1000µg will be despatched as 2 x 500µg
Fast and free shipping.
Buy online with secure credit card or purchase order. For any questions, please email orders@qkine.com
Summary:
- High-purity human GDF-5 (UniProt number: P43026)
- 27 kDa (monomer)
>98%, by SDS-PAGE quantitative densitometry
Expressed in E. coli
Animal origin-free (AOF) and carrier protein-free
Manufactured in our Cambridge, UK laboratories
Lyophilized from acetonitrile, TFA
- Resuspend in 10 mM HCl (Reconstitution solution A) at >50 µg/ml, add carrier protein if desired, prepare single-use aliquots and store frozen at -20 °C (short-term) or -80 °C (long-term)
Featured applications:
Directed chondrogenic differentiation of mesenchymal stem cells
Generation of iPSC-derived osteoblasts and chondrocytes
Mesenchymal stem cell research
Differentiation of adipose-derived stromal cells to osteogenic lineages
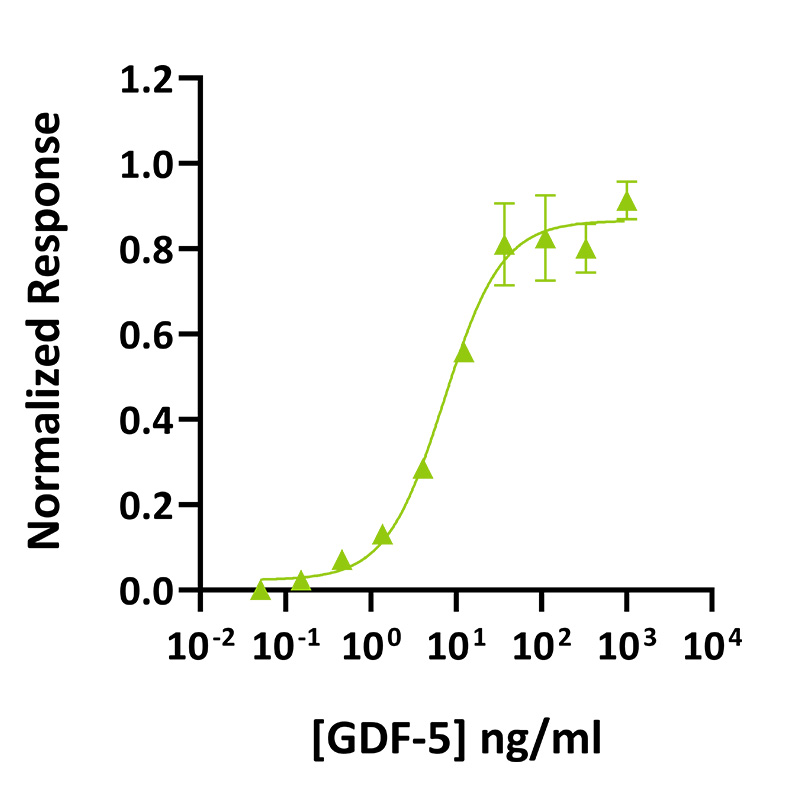
GDF-5 activity was determined using the GDF-5-responsive firefly luciferase reporter assay. Transfected HEK293T cells were treated in triplicate with a serial dilution of GDF-5 for 24 hours. Firefly luciferase activity was measured and normalised to the control Renilla luciferase activity. Data from Qk070 lot #204571. EC50 = 7.2 ng/mL (0.53 nM).
Recombinant GDF-5 migrates as a major band at approximately 27 kDa in non-reduced conditions (NR). Upon reduction (R), only the monomer band at approximately 13.5 kDa is visible. No contaminating protein bands are present. The purified recombinant protein (3 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptoethanol, R) and non-reduced (NR) conditions and stained with Coomassie Brilliant Blue R250. Data from Qk070 batch #204571.

Further quality assays
Mass spectrometry: single species with expected mass
Recovery from stock vial: >95%
Endotoxin: <0.005 EU/μg protein (below level of detection)
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.

Qkine GDF-5 is as biologically active as a comparable alternative supplier protein. Bioactivity was determined using a GDF-5-responsive firefly luciferase reporter assay. Stably transfected HEK293T cells were treated in triplicate with a serial dilution of Qkine GDF-5 (Qk070, green) or an alternative supplier (Supplier B, black) for 24 hours. Firefly luciferase activity was measured and normalised to the control Renilla luciferase activity. Data from Qk070 lot #204559.
Technote | GDF-5 (Qk070) bioactivityProtein background
Growth differentiation factor 5 (GDF-5) belongs to the transforming growth factor beta (TGF-β) superfamily and is closely related to the bone morphogenetic proteins (BMPs) subfamily [1]–[4]. GDF-5 plays a crucial role during embryonic development and tissue homeostasis and is specifically involved in the development of the skeletal system, where it promotes the formation and maintenance of bones and joints [1], [2]. GDF-5 regulates the proliferation and differentiation of the osteoblasts (osteogenesis) and chondrocytes (chondrogenesis)[2].
GDF-5, like other members of the TGF-β superfamily, is characterized by a conserved structure consisting of a signal peptide, prodomain, and a mature domain [5]. The mature domain, responsible for the biological activity of the protein, contains a cysteine knot motif formed by six conserved cysteine residues [5]. GDF-5 binds to membrane-bound serine-/threonine-kinase receptors, termed type I and type II receptors, regulating downstream intracellular signaling pathways similarly to other members of TGF-β superfamily [2], [5]. GDF-5 activates Smad-dependent and Smad-independent pathways (MAPK, PI3K, and Wnt/β-catenin pathways) to regulate cellular processes such as development, growth, and survival [5]–[8].
GDF-5 is commonly in cell culture for the differentiation and maintenance of induced pluripotent stem cells, embryonic stem cells, or bone marrow-derived mesenchymal stem cells. It promotes the differentiation to osteoblasts and chondrocytes when used with BMP-4 [1], [2], [6], [9], [10]. Additionally, GDF-5 is used with TGF-β1 to stimulate osteogenic differentiation of adipose-derived stromal cells [11], [12].
Mutations in the GDF-5 gene have been associated with various skeletal disorders [3], [5], [13]. For example, loss-of-function mutations in GDF-5 have been linked to congenital disorders such as Hunter-Thompson syndrome, brachydactyly type C, and DuPan syndrome [14]–[17]. On the other hand, polymorphisms in the GDF-5 gene have been associated with an increased risk of developing osteoarthritis, a degenerative joint disease [3], [13]. Manipulating GDF-5 activity could offer novel therapeutic strategies for promoting bone and joint regeneration or for mitigating the progression of diseases affecting these tissues.
Additional resources
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.

Receive an Amazon gift voucher when you leave us a review.
£25, $30 or €30 for reviews with an image and £10, $15 or €15 for reviews without an image