Differentiation of induced pluripotent stem cells (iPSCs) into microglia
Leighton Sneade
Qkine
Introduction
Microglia, which originate from mesodermal and mesenchymal lineage, serve as the primary innate immune cells of the central nervous system (CNS) [1]. These cells are highly dynamic and capable of altering their morphology and function in response to various neurological challenges. They play crucial roles in synaptic plasticity, neurogenesis, maintaining homeostasis, and immune defense within the CNS [2] Microglia have also been increasingly recognized for their involvement in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and adrenoleukodystrophy (ALD) [3].
Studying human microglia has historically been challenging due to the difficulty of obtaining primary cells from human donors. To overcome this limitation, deriving microglia from induced pluripotent stem cells (iPSCs) has emerged as a promising solution. iPSCs, generated by reprogramming somatic cells into a pluripotent state, can be differentiated into various cell types, including microglia [4]. This approach provides an accessible, scalable, and versatile platform for investigating microglial biology, modeling neurological diseases, and exploring therapeutic applications.
Differentiating iPSCs into microglia involves activating key signaling pathways that mimic the embryonic development of microglia from yolk sac progenitors. The CSF1R signaling pathway, activated by IL-34, is crucial for microglial survival, proliferation, and differentiation. This receptor regulates downstream processes that guide progenitors into the microglial lineage. The TGF-β pathway promotes microglial maturation and the acquisition of functional phenotypes relevant to their roles in the CNS.
Methods
Cell culture and maintenance
iPSCs were passaged twice per week using 0.5 mM EDTA for detachment and seeded in vitronectin-coated (Qk120), 5 µg/ml) 6-well plates using a 1:6 split ratio and cultured in an E8-like medium. The day after passage, spent medium was removed and replaced with 5 ml of fresh E8-like medium, enabling a weekend-free media change schedule. For further information on this process, please see our guide to Weekend-free human induced pluripotent stem cell culture using thermostable FGF-2 (bFGF) from Qkine, in combination with our animal origin-free TGF-β1 (Qk010) and vitronectin, for improved colony homogeneity.
iPSC differentiation into Microglia
iPSC plating (Day 0)
iPSCs were detached using AccutaseTM and seeded at 3.15×105 cells/well in a Matrigel-coated (0.5 mg/ml) 6-well plate in E8-like medium containing ROCK inhibitor (Y-27632, 10 µM).
Mesoderm Induction (Days 1-4)
For each well, the medium was replaced daily with 2 ml of mesoderm Induction medium (Table 1) to initiate differentiation towards the mesodermal lineage.
| Component | Final Concentration |
|---|---|
| DMEM/F12 medium (Thermo Fisher Scientific,31330038) | 1x |
| Sodium bicarbonate (Merck, S5761) | 543 mg/l |
| Sodium chloride (Merck, S5886) | 1000 mg/l |
| L-ascorbic acid (Merck, A8960) | 64 mg/l |
| Insulin-Transferrin-Selenium (ITS-G) (Thermo Fisher Scientific, 41400045) | 1x |
| BMP-4 (Qkine, Qk038) | 80 ng/ml |
Table 1. Mesoderm induction medium composition. Components added aseptically and filter sterilized before use.
Hematopoietic Specification (Days 5-6)
Medium was replaced daily with 2 ml of fresh hematopoietic specification medium (Table 2), to promote the differentiation of mesodermal cells into hemangioblasts.
| Component | Final Concentration |
|---|---|
| Gibco™ StemPro™-34 SFM (1X) (Thermo Fisher Scientific,11580356) | 1x |
| Gibco™ GlutaMax™ Supplement (Thermo Fisher Scientific, 11584466) | 2 mM |
| FGF2-G3 (Qkine, Qk052) | 25 ng/ml |
| SCF (Qkine, Qk078) | 100 ng/ml |
| VEGF 165 (Qkine, Qk048) | 80 ng/ml |
Table 2. Hematopoietic specification medium composition. Components added aseptically and filter sterilized before use.
Myeloid Progenitor Expansion (Day 7-14)
Medium was replaced daily with 2 ml of fresh myeloid expansion medium (Table 3), to promote differentiation into myeloid progenitors.
To retain detached maturing cells, from day 10 until day 14, the supernatant was collected daily and centrifuged at 300 xg for 6 minutes. After centrifugation, the supernatant was aspirated and the cell pellet resuspended in fresh medium, which was then returned to its original well.
| Component | Final Concentration |
|---|---|
| Gibco™ StemPro™-34 SFM (1X) (Thermo Fisher Scientific,11580356) | 1x |
| Gibco™ GlutaMax™ Supplement (Thermo Fisher Scientific, 11584466) | 2 mM |
| IL-3 (Qkine, Qk090) | 50 ng/ml |
| SCF (Qkine, Qk078) | 50 ng/ml |
| M-CSF (Qkine, Qk075) | 50 ng/ml |
| Flt3L (Qkine, Qk087) | 50 ng/ml |
| Thrombopoietin (TPO) (Peprotech, 300-18-10UG)* | 5 ng/ml |
Table 3: Myeloid expansion medium composition. *Following the generation of the data in this application note, Qkine have introduced a high-purity, animal-origin-free TPO (Qk098), which offers a suitable alternative to the TPO used.
Microglial Progenitor Maturation (Day 15-25)
The medium was changed every four days (on days 15, 19, and 23) with 2 ml of Microglial Maturation medium (Table 4) to promote the maturation of myeloid progenitors into microglial progenitors. During each medium change, the supernatant from the wells was collected and centrifuged at 300 xg for 6 minutes. The resulting supernatant was then aspirated, and the cell pellet was resuspended in fresh medium and returned to its original well.
| Component | Final Concentration |
|---|---|
| Gibco™ StemPro™-34 SFM (1X) (Thermo Fisher Scientific,11580356) | 1x |
| GM-CSF (Qkine, Qk076) | 25 ng/ml |
| M-CSF (Qkine, Qk075) | 50 ng/ml |
| Flt3L (Qkine, Qk087) | 50 ng/ml |
Table 4: Microglial maturation medium composition. Components added aseptically and filter sterilized before use.
Isolation of Microglial Progenitors (Day 26+)
Supernatant from each well was collected and centrifuged at 300 xg for 6 minutes. Following centrifugation, the supernatant was aspirated, and the cell pellet was resuspended in an appropriate volume (approx. 2 ml) of fresh medium and counted. These cells were then seeded at 9.5×105 cells/well in a Matrigel-coated (0.5 mg/ml) 6-well plate in SF-Microglial medium (Table 5).
| Component | Final Concentration |
|---|---|
| Gibco™ RPMI-1640 with 2 mM GlutaMAX-I (Thermo Fisher Scientific,11569726) | 1x |
| GM-CSF (Qkine, Qk076) | 10 ng/ml |
| IL-34 PLUSTM (Qkine, Qk091) | 100 ng/ml |
Table 5: SF-Microglial medium composition. Components added aseptically and filter sterilized before use.
To promote additional maturation of myeloid progenitors into microglial progenitors, the original 6-well plate culture can be continued with medium changed every four days with 2 ml of microglial maturation medium (Table 4). The culture can be sustained for up to approximately 50 days, with further isolations carried out as needed during this time.
Differentiation into Mature Microglia (Post-Isolation of Microglial Progenitors)
The progenitor cells were differentiated into microglia in 2 ml of SF-Microglial medium per well (Table 5), with medium changes every 3 to 4 days over a two-week period.
Immunocytochemistry
Microglial cells were fixed with 4% paraformaldehyde, then blocked and permeabilized with 10% donkey serum diluted in 0.1% Triton X-100. Specific antibodies targeting microglial markers IBA-1 and P2RY12 were applied for immunostaining overnight at 4°C. Cells were then washed and incubated with the secondary antibodies Donkey anti-Goat AlexaFluorTM 488, Donkey anti-Rabbit AlexaFluorTM 597, and Hoechst 33258, followed by imaging in phosphate buffered saline.
Results
Immunocytochemistry analysis demonstrated that the iPSC-derived cells acquired a microglial phenotype. After differentiation, cultures contained cells with small cell bodies and fine, highly branched processes, consistent with the ramified morphology characteristic of microglia in vitro. Marker staining confirmed expression of microglia-specific proteins: IBA1 labeling was detected throughout the cytoplasm, while P2RY12 was localized to the cell body and membranes, providing clear evidence of microglial identity (figures 1 and 2).
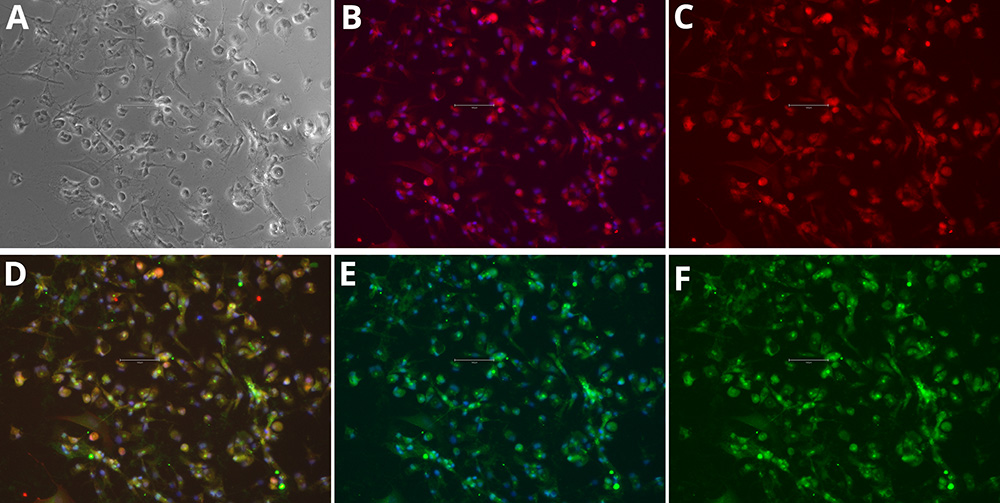
Figure 1. Immunocytochemistry of microglia markers in differentiated iPSCs. Phase Contrast [A], P2RY12 and Hoechst 33258 [B], P2RY12 [Red, C], IBA-1, P2RY12 and Hoechst 33258 [D], IBA-1 and Hoechst 33258 [E], IBA-1 [Green, F]. Images were acquired using the EVOS M5000 system (scale bar = 150 µm).
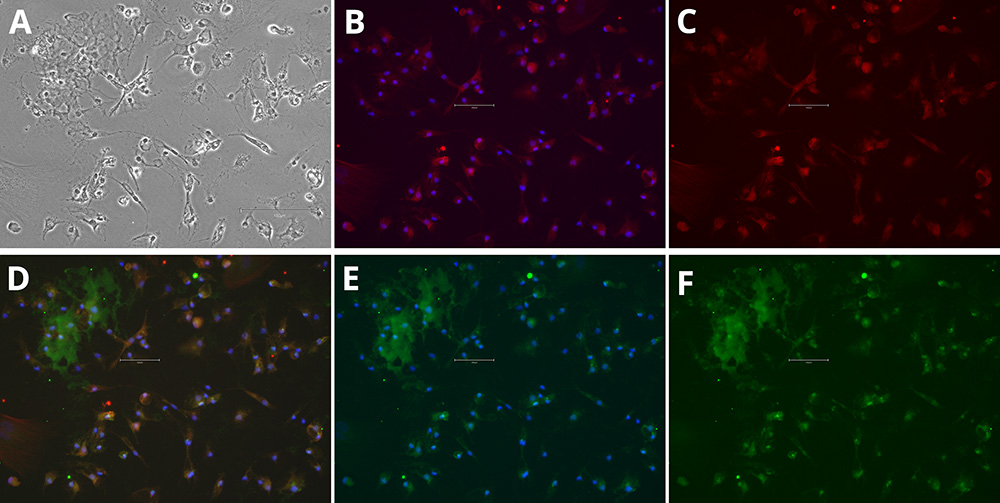
Figure 2. Immunocytochemistry of microglia markers in differentiated iPSCs. Phase Contrast [A], P2RY12 and Hoechst 33258 [B], P2RY12 [Red, C], IBA-1, P2RY12 and Hoechst 33258 [D], IBA-1 and Hoechst 33258 [E], IBA-1 [Green, F]. Images were acquired using the EVOS M5000 system (scale bar = 150 µm).
The co-localization of these markers within the same cells further supports a microglia-like profile. Together, the morphological features and marker expression patterns confirm that the differentiation protocol reliably produced cells with microglial characteristics suitable for downstream studies.
Conclusion
The growing significance of iPSCs in clinical and research applications stems from their unique ability to differentiate into all somatic cell types in the human body. Successful differentiation into specific lineages, such as microglia, depends on maintaining pluripotency during culture and achieving homogenous, reproducible differentiation. The use of optimized medium containing highly pure and bioactive growth factors is critical to driving efficient, high-quality differentiations and outcomes.
The data presented in this application note demonstrate that high purity, animal origin-free growth factors from Qkine – including BMP-4 (Qk038), FGF2-G3 (Qk052), SCF (Qk078), VEGF 165 (Qk048), IL-3 (Qk090), M-CSF (Qk075), Flt3 ligand (Qk087), IL-34 PLUS TM (Qk091) and GM-CSF (Qk076) – support robust and reliable differentiation of iPSCs into microglial cells. These growth factors reduce variability and enhance reproducibility, which are essential for both research and therapeutic applications.
Differentiation was sustained over a long-term culture period of up to 50 days, with medium changes up to every four days and additional cell isolations performed during this time. Mature microglia were confirmed by immunocytochemistry, showing expression of key markers IBA1 and P2RY12. The use of a weekend-free culture system, enabled by thermostable FGF-2 (Qk052) and used with animal origin-free vitronectin (Qk120) and TGF-β1 (Qk010), further contributed to consistent colony morphology and reduced handling variability.
Following the generation of the data in this application note, we have introduced a high-purity, animal-origin-free TPO (Qk098), which offers a suitable alternative to the TPO previously used in this process.
This work highlights the importance of high-quality reagents in overcoming challenges associated with iPSC differentiation, such as inconsistent cell performance or variability in outcome. By using Qkine’s portfolio of defined, animal origin-free growth factors from Qkine, researchers can improve the reproducibility of complex, long-term cell cultures and advance applications in regenerative medicine, drug discovery, and disease modeling.
Further information
Qkine growth factors are manufactured to the highest quality standards and are free from animal-derived contaminants, delivering low endotoxicity and high purity. At Qkine, we are committed to raising the standards of growth factors, cytokines and related proteins to better support long-term and complex cell cultures. We are a science-led team, please reach out with any questions or requests to support@qkine.com.
The differentiation protocol was developed following methodology described in Douvaras P, et al., 2017 [5]. We make no claim to novelty in the methodology and acknowledge the intellectual property held by the original inventors.
References
[1] Woodburn SC, Bollinger JL and Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation 18, 258 (2021). https://doi.org/10.1186/s12974-021-02309-6
[2] Abud EM, Ramirez RN, Martinez ES et al. iPSC-Derived Human Microglia-like Cells to Study Neurological Diseases. Neuron. (2017). doi: 10.1016/j.neuron.2017.03.042.
[3] Muffat J, Li Y, Yuan B et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med 22 (2016). https://doi.org/10.1038/nm.4189
[4] Chen SW, Hung YS, Fuh JL et al. Efficient conversion of human induced pluripotent stem cells into microglia by defined transcription factors. Stem Cell Reports. (2021). doi: 10.1016/j.stemcr.2021.03.010.
[5] Douvaras P, Sun B, Wang M, et al. Directed Differentiation of Human Pluripotent Stem Cells to Microglia. Stem Cell Reports. (2017) doi: 10.1016/j.stemcr.2017.04.023.
All our recombinant proteins are animal-free and come with Bioactivity. Guaranteed.
Contact us
Our science team is here to help, please contact us if you have any questions.