 Recombinant bovine/porcine activin A protein (Qk108-FG)
Recombinant bovine/porcine activin A protein (Qk108-FG)Recombinant bovine/porcine activin A protein (Qk108-FG)
Price range: £230.00 through £2,900.00
Activin A is a TGF-β family growth factor regulating embryonic development, cell proliferation, differentiation, and immune responses. Food grade activin A is frequently used to maintain pluripotency in induced pluripotent and embryonic stem cell cultures for cultivated meat and fat.
High quality food grade recombinant bovine/porcine activin A protein is a high-purity mature bioactive dimer of 26 kDa. It is animal origin-free, carrier protein-free, and tag-free to ensure its purity with exceptional lot-to-lot consistency.
In stock
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
Price range: £230.00 through £2,900.00
Buy online with secure credit card or purchase order. For any questions, please email orders@qkine.com
Summary:
- Bioactive mature domain of recombinant bovine/porcine activin A protein (Uniprot: P07995)
- 26 kDa (dimer)
High quality food grade recombinant protein
>98%, by SDS-PAGE quantitative densitometry
Animal origin-free (AOF) and carrier protein-free
Expressed in E. coli
Manufactured in the UK under a food manufacturing HACCP regime
Lyophilized from acetonitrile, TFA
- Resuspend in 10 mM HCl (Reconstitution solution A) at >50 µg/ml, add carrier protein if desired, prepare single-use aliquots and store frozen at -20 °C (short-term) or -80 °C (long-term)
Featured applications:
Cellular agriculture and cultivated meat cell culture media optimization
Cellular agriculture process development
Induced pluripotent stem cell culture and maintenance
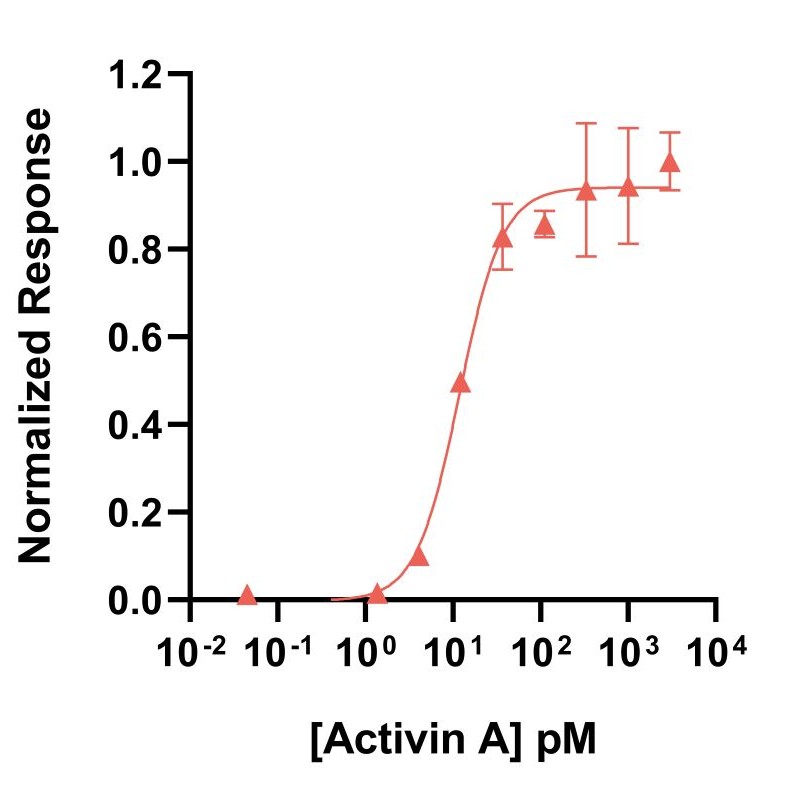
Activin A activity is determined using the activin-responsive firefly luciferase reporter assay in stably transfected HEK293T cells. Cells are treated in triplicate with a serial dilution of activin A. Firefly luciferase activity is measured and normalized. EC50 = 11.9 pM (309.4 pg/ml). Data from Qk001 lot #104276.
Activin A migrates as a single band at 24 kDa in non-reducing (NR) and 13 kDa as a single monomeric species upon reduction (R). No contaminating protein bands are visible. Purified recombinant protein (7 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced conditions (NR) and stained with Coomassie Brilliant Blue R250. Data from Qk001 lot #011.

Further quality assays
Mass spectrometry: single species with expected mass
Recovery from stock vial: >95%
Endotoxin: <0.005 EU/μg protein (below level of detection)
Full raw materials traceability, allergen analysis, CoO, CoA, beta-lactam-free and animal origin-free certification available
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
Activin A is a member of the belongs to the transforming growth factor beta (TGF-β) superfamily TGF-β family of growth factors [1]–[4]. It was first identified for its ability to stimulate the release of follicle-stimulating hormone (FSH) from the pituitary gland. It was later recognized as a multifunctional protein with diverse cellular effects [5], [6]. It plays crucial physiological roles in regulating embryonic development, cell proliferation, and differentiation [6], [7]. It promotes the patterning and differentiation of various organs, including the development of the mesoderm, neural, and reproductive systems. It is also involved in maintaining homeostasis, regulating immune responses, and wound healing [8]. Impaired activin A signaling has been associated with various pathological conditions, including cancer, inflammation, and fibrosis [9], [10]. As activin A can contribute to disease progression and severity, it is a growing area of research for promising therapeutic targets.
Activins are disulfide-linked homo- and heterodimers of four inhibin β chains [6]. The best-characterized are activin A and activin B, homodimers of inhibin βA and inhibin βB, respectively. Like all other members of the TGF-β family, activins are synthesized as significant precursors consisting of an N-terminal signal peptide, a pro-domain of 250–350 residues, and a highly conserved mature domain. The pro-domain, which is cleaved off in the mature protein, has essential roles in the biosynthesis, stabilization, transportation and signaling of the growth factors in the body [11]. Activin A binds to activin type I (ALK4 or ALK7) and type II (ActRIIA or ActRIIB) receptors [6], [12]. The type II receptor phosphorylates the type I receptor upon ligand binding, initiating downstream signaling cascades, mainly through the SMAD family of proteins. Activated SMAD complexes translocate into the nucleus, regulating the transcription of target genes involved in various cellular processes. In vivo, the high-affinity inhibitor follistatin and inhibins tightly regulate activin A activity in a feedback loop [13], [14]. Follistatin is secreted into the media during stem cell culture. However, the impact on the efficiency of stem cell differentiation and cellular homogeneity has not been studied closely (see this discussion for more information).
Activin A is frequently used for the maintenance of pluripotency of human induced pluripotent stem cells and human embryonic stem cell lines along with fibroblast growth factor 2 (FGF-2) [2], [4]. Activin A is also used in various stem cell differentiation protocols. It directs the differentiation into definitive endoderm, precursor to different cell types such as pancreatic and liver cells [15]–[18]. Activin A also promotes neural precursor cells and drives astrocytic differentiation with Ciliary neurotrophic factor (CNTF) and Glial cell line-derived neurotrophic factor (GDNF) [19]. Moreover, activin A is also involved in mesodermal differentiation to derive muscle, bone, and blood cells. Finally, activin A is often used for the development and maintenance of organoids [18], [20], [21]. Qkine recombinant activin A protein has been extensively validated and benchmarked with other suppliers’ proteins in stem cell culture and assays. You can view the results of this analysis and a commentary by Qkine’s founder, Marko Hyvönen.
FAQ
Activin A is a multifunctional protein. It is involved in regulating embryonic development, cell proliferation, and differentiation. It promotes the patterning and differentiation of various organs, including the development of the mesoderm, neural, and reproductive systems. It is also involved in maintaining homeostasis, regulating immune responses, and wound healing. Finally, it stimulates the release of follicle-stimulating hormone from the pituitary gland.
Activin A is a critical factor in stem cell culture, commonly used to maintain the pluripotency of induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs). It is integral to various stem cell differentiation protocols, guiding the differentiation of the definitive endoderm, neural and mesodermal lineages. Activin A is also commonly utilized in the development and maintenance of organoids.
Activin A and inhibin A are closely related proteins in the TGF-β superfamily. They share structural similarities but have distinct functions and roles in regulating various physiological processes. Activin A has broader functions beyond reproduction, which include handling embryonic development, stem cell maintenance, and the immune system. Inhibin A, conversely, is more specifically associated with the feedback control of follicle-stimulating hormones in the context of reproductive physiology.
Activin A binds to activin type I (ALK4 or ALK7) and type II (ActRIIA or ActRIIB) receptors to activate downstream SMAD signalling.
The activin gene family includes several genes that encode different activin subunits, forming various activin isoforms such as Inhibin Beta Subunits (INHBA / INHBB / INHBC / INHBD).
Follistatin and inhibins tightly regulate activin A activity in a feedback loop.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.
For use in manufacturing of cellular or gene therapy products. Not intended for in vivo applications.

Receive an Amazon gift voucher when you leave us a review.
£25, $30 or €30 for reviews with an image and £10, $15 or €15 for reviews without an image
