Currency
Recombinant human G-CSF protein (Qk074)
Granulocyte colony-stimulating factor (G-CSF) is a member of the hematopoietic growth factor family which plays a crucial role in the proliferation, differentiation, and maturation of committed progenitor cells to granulocytes, such as neutrophils.
Qkine G-CSF is a highly pure, bioactive glycoprotein produced in an animal origin-free expression system composed of 174 amino acids with a molecular weight of 18.6 kDa. Human and murine G-CSF are 73% identical at the amino acid level and show species cross-reactivity. Human G-CSF is carrier-free, tag-free, and non-glycosylated to ensure a pure and homogenous production of high-quality neutrophils and other relevant cell cultures with exceptional lot-to-lot consistency.
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
1000µg will be despatched as 2 x 500µg
Fast and free shipping.
Buy online with secure credit card or purchase order.
For any questions, please email orders@qkine.com
Summary
High purity human G-CSF protein (Uniprot: P09919-2)
>98%, by SDS-PAGE quantitative densitometry
- Expressed in E. coli
- 18.6 kDa monomer
Animal origin-free (AOF) and carrier protein-free
- Manufactured in Cambridge, UK
- Lyophilized from Tris/mannitol
Resuspend in water at >100 µg/ml, prepare single-use aliquots, add carrier protein if desired, and store frozen at -20°C or -80°C
Featured applications
- Generation of iPSC-derived neutrophils
- Induction of cardiomyocytes from iPSCs
- Mobilization of bone marrow cells for wound healing applications
- Regulation of ZO-1 expression in brain microvascular endothelial cells
- Proliferation of neutrophils
- Maintenance of isolated neutrophils
Granulocyte colony-stimulating factor, G-CSF, GCSF, GM-CSF beta, Colony-stimulating factor 3, CSF3, CSF-3, CSF3OS, Pluripoietin, Filgratin, Lenogratin, C17orf33, chromosome 17 open reading frame 33, MGC45931
human
species similarity:
mouse – 75%
rat – 73%
porcine – 79%
bovine – 81%
Bioactivity
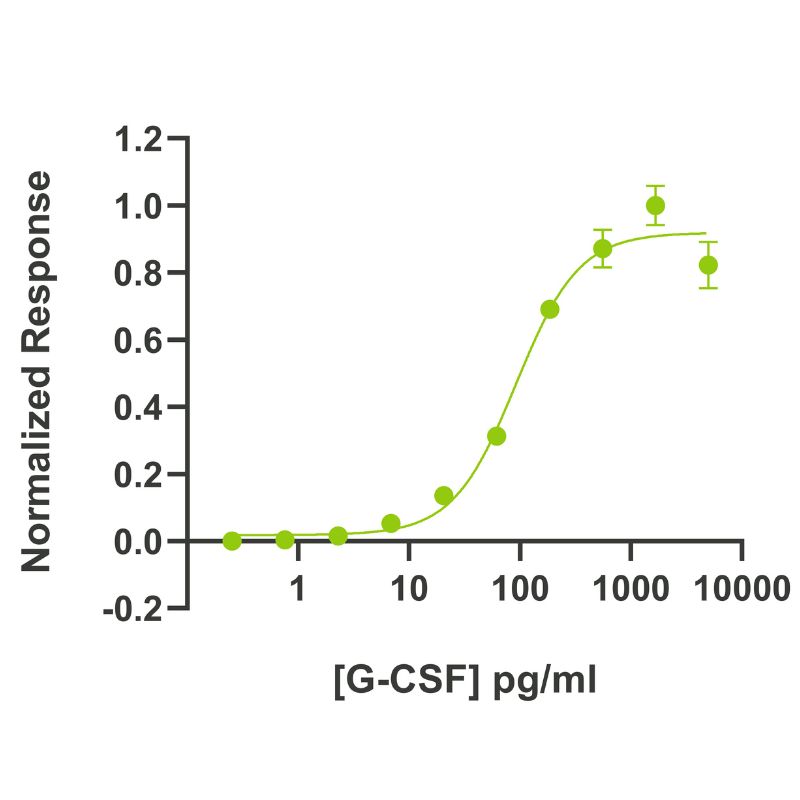
G-CSF activity is determined using the Promega CellTiter-Glo luminescent cell viability assay. EC50 = 92 pg/ml (4.9 pM). NFS-60 mouse myeloid leukemia cells are treated in triplicate with a serial dilution of G-CSF for 48 hours. Cell viability is measured and normalized. Data from Qk074 lot #204487.
Purity
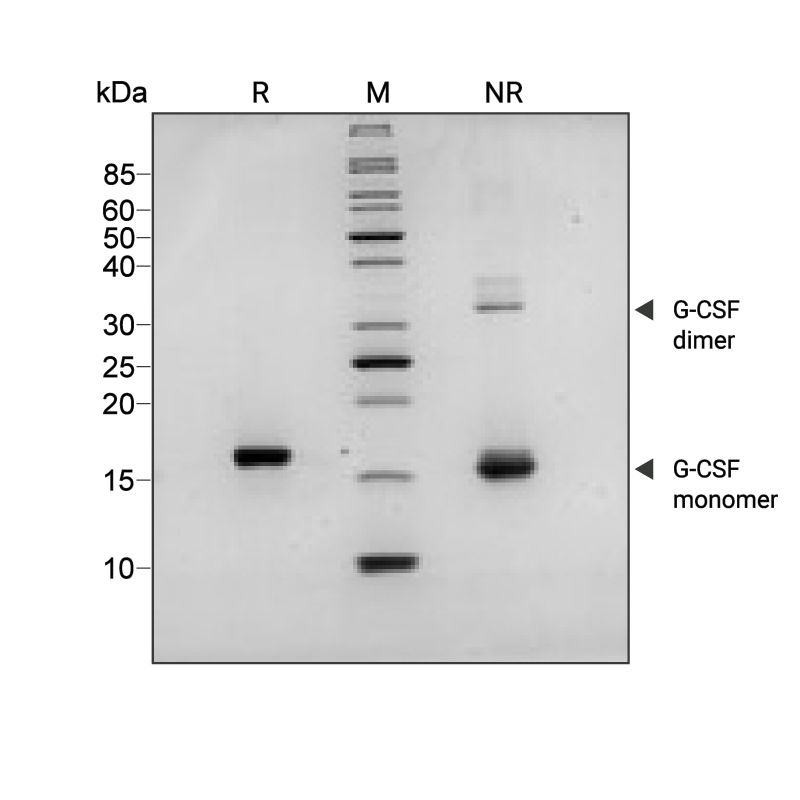
Recombinant G-CSF migrates as a major band at approximately 18 kDa (monomer) in non-reducing (NR) conditions. The dimeric form is the minor band at the higher molecular weight (36 kDa). Upon reduction (R), only the 18 kDa band is visible. No contaminating protein bands are present. The purified recombinant protein (3 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced (NR) conditions and stained with Coomassie Brilliant Blue R250. Data from Qk074 batch #204487.
Further quality assays
- Mass spectrometry, single species with the expected mass
- Endotoxin: <0.005 EU/μg protein (below the level of detection)
- Recovery from stock vial: >95%
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
Granulocyte colony-stimulating factor (G-CSF), or colony-stimulating factor 3 (CSF-3), is a secreted glycoprotein belonging to the family of hematopoietic growth factors. It regulates granulopoiesis leading to the maturation of committed progenitors to granulocytes, such as neutrophils [1–3]. G-CSF is involved in the proliferation, maturation, and mobilization of neutrophils in both the healthy and diseased states [3,4]. It was one of the first cytokines to be identified in vitro and was successfully isolated from human cells in 1984 [4–7].
Human G-CSF is composed of 174 amino acids with a molecular weight of 18.6 kDa [3]. G-CSF can be released by various cells such as macrophages, fibroblasts, endothelial cells, and epithelial cells [4] under inflammatory mediators’ stimulation such as interleukin (IL)-17 and IL-1, tumor necrosis factor (TNF)-α, interferon (IFN)-β, lipopolysaccharide (LPS) as well as vascular endothelial growth factor (VEGF) [8–10].
G-CSF binds to the homodimer GCSF receptor (GCSF-R) expressed on myeloid cells as well as fibroblasts, endothelial cells, and bone marrow stromal cells [2,11]. GCSF-R is an 813-amino acid protein and is composed of an extracellular region, which consists of an immunoglobin-like domain, a cytokine receptor homologous domain, three fibronectin type III-like domains, a transmembrane region, and an intracellular region [1]. G-CSF binds to G-CSFR, resulting in its dimerization and the activation of downstream signaling pathways. Among the activated downstream signal transduction pathways are Janus kinase (JAK)/signal transducer and activator of transcription (STAT), Src kinases such as Lyn, Ras/Extracellular Regulated Kinase (ERK), and phosphatidylinositol 3-kinase (PI3K).
G-CSF has been used in vitro to maintain neutrophils and stimulate the differentiation of cells to neutrophil lineage [12] such as generating functional neutrophils from iPSCs [13,14]. In addition, G-CSFR was shown to contribute to the activation of STAT signaling and cardiac differentiation from iPSCs [15].
G-CSF has been shown to have aberrant expression in some disorders such as Alzheimer’s disease. A study by Zhang et al. reported that G-CSF downregulates ZO-1 expression in brain microvascular endothelial cells highlighting a potential therapeutic target for Alzheimer’s disease [16].
In vivo, G-CSF was shown to contribute to the improvement of memory and neurobehavioral function in an amyloid-beta-induced experimental model of Alzheimer’s disease [17]. In wound healing applications, G-CSF has been shown to have a crucial role in mobilizing bone marrow-derived cells to accelerate the wound healing process [18]. Hence, it is administered following bone marrow transplantation to facilitate hematopoietic recovery. Finally, G-CSF is used as a therapeutic agent in neutropenia, a condition resulting in a low number of neutrophils in the blood, particularly in patients with cancer undergoing chemotherapy [4,19].
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.



What others are saying
There are no contributions yet.