Sensitive methods for the confirmation of protein identity
At Qkine our in-house R&D team test all our cytokines and growth factors to ensure their form and identity.
Mass spectrometry analysis is used to confirm the molecular mass of the intact protein and to reveal any heterogeneity that would not be evident from SDS-PAGE analysis. The resultant mass is compared with the calculated mass of the protein with the assumption that all the cysteines are disulfide-linked. Multiple peaks represent different charge states of the protein.
We carry out mass spectrometry analysis on every lot to ensure consistent lot-to-lot identity and purity.
![Mass-spectrometry-analysis Qkine Mass spectrometry analysis [LC-MS]](https://421795589.r.directcdn.net/wp-content/uploads/2023/10/Mass-spectrometry-analysis-400x225.jpg)
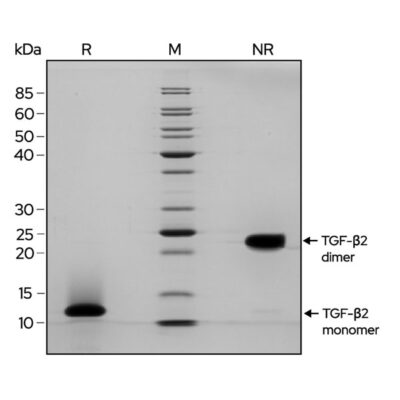
For proteins such as the TGF-beta family the active form is dimeric, SDS-PAGE is use to ensure the protein is in the pure dimeric form to ensure maximum bioactivity.
For example, recombinant TGF-β2 (Qk072) migrates as a major band at approximately 25 kDa (dimer) in non-reducing (NR) conditions. Upon reduction (R), the monomer band at approximately 12.5 kDa is visible.
Getting the simple things right is important.
At Qkine, we are committed to raising the standard in bioactive protein manufacturing.
Our science team is here to help, please contact us if you have any questions at customerservice@qkine.com.
SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a method that separates proteins by mass. SDS is an ionic detergent that denatures and binds to proteins to make them uniformly negatively charged. This means that when an electronic current is applied to a polyacrylamide gel, the SDS-bound proteins will migrate down the gel towards the positively charged electrode, separated by size alone.
Proteins can be reduced before being run on an SDS-PAGE gel. In reducing conditions β-mercaptoethanol (β-ME or 2-ME) or dithiothreitol (DTT) is added; this reduces the disulfide bridges in proteins so that when they are run on the gel, they are better separated by size. Many of our growth factors are disulfide-linked dimers so running non-reduced proteins on the same gel as reduced proteins allows confirmation of the correct dimeric state.
As standard, we run all 3µg and 7µg of our recombinant proteins in reduced and non-reduced conditions to ensure there are no contaminants, aggregates or degradants.