Stringent quality control
Our robust animal-free manufacturing platform, along with rigorous quality control procedures, ensures exceptional bioactivity and consistent performance from lot to lot, guaranteeing outstanding performance in your applications.
Providing the highest-quality growth factors and cytokines
To ensure that your results are reliable and reproducible every time, we have stringent quality control procedures in place to provide you with highly pure proteins with exceptional bioactivity, providing you with outstanding performance in your cultures.
We couple this with unrivaled lot-to-lot consistency, ensuring every single batch meets the same quality standards as all previous ones, thereby reducing the need for batch testing.
Our promise
Purchase with confidence with our Bioactivity Guarantee
We are confident that the quality and bioactivity of our recombinant proteins will ensure that you achieve your research goals. However, if for any reason you experience an issue with your Qkine recombinant protein, please contact us immediately, and we will refund or replace your protein.
Our expert team of stem cell scientists and protein biochemists will then work with you to ensure you achieve your desired results.

Our stringent quality control explained
Endotoxin assay
Endotoxins are small hydrophobic lipopolysaccharide molecules, toxic substances found in the outer cell membrane of gram-negative bacteria. Endotoxins are shed by bacteria during their cell death or when they are actively growing and dividing. Endotoxins can affect the growth or performance of cell cultures and are a major source of experimental variation. Endotoxins have a very high heat stability meaning they cannot be destroyed with regular decontamination methods such as autoclaving. Endotoxins are also hydrophobic, and consequently have a strong affinity for other hydrophobic materials such as lab plastics. Endotoxins can be avoided by cleanliness and proper lab techniques to keep endotoxin levels in a lab at bay. At Qkine, we achieve this with good aseptic technique, filter sterilization of buffers used during manufacturing processes, and rigorous purification column cleaning protocols. This minimizes the introduction of endotoxins into any manufacturing/research processes.
To determine the presence of endotoxins, a Limulus Amebocyte Lysate (LAL) assay is used. This assay can detect as little as 0.01 endotoxin units (EU)/mL. One EU equals approximately 0.1 to 0.2 ng endotoxin/mL of solution. Current industry standards require endotoxin levels in growth factor lots to be less than 0.5 EU/mL, however the lower the better, and companies such as Qkine impose internal standards of <0.1 EU per µg protein.
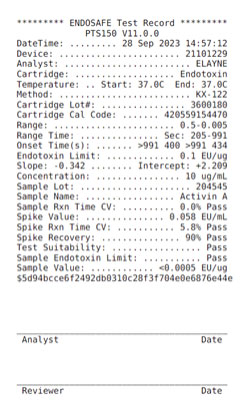
Example result
Mass spectrometry analysis [LC-MS]
Mass spectrometry analysis is used to confirm the molecular mass of the intact protein and to reveal any heterogeneity that would not be evident from SDS-PAGE analysis. The resultant mass is compared with the calculated mass of the protein with the assumption that all the cysteines are disulfide-linked. Multiple peaks represent different charge states of the protein. We carry out mass spectrometry analysis on every batch.
![Mass-spectrometry-analysis Qkine Mass spectrometry analysis [LC-MS]](https://qkine.com/wp-content/uploads/2023/10/Mass-spectrometry-analysis.jpg)
Example result
UV recovery
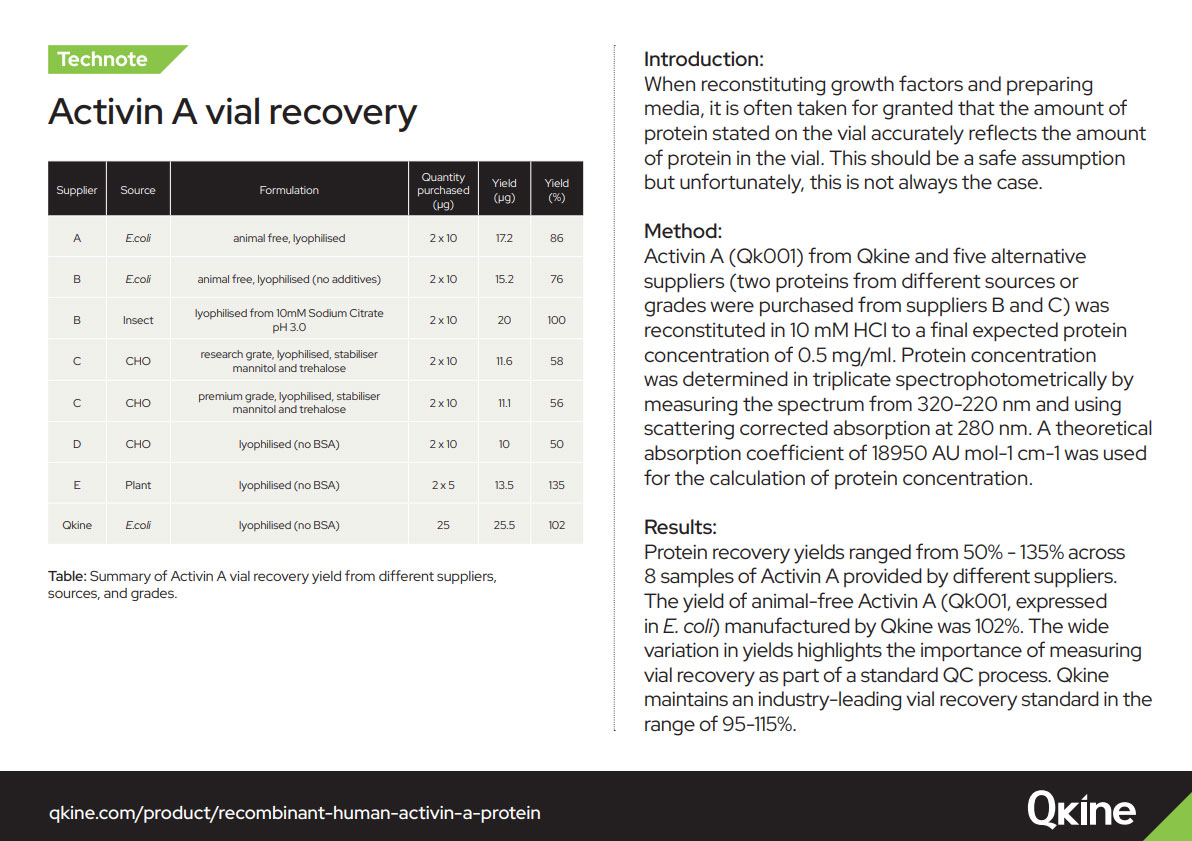
When reconstituting growth factors and preparing media, it is often taken for granted that the amount of protein stated on the vial accurately reflects the amount of protein in the vial. This should be a safe assumption but unfortunately, this is not always the case. At Qkine, we check the vial recovery on every batch using UV spectrophotometry to ensure that we maintain an industry-leading vial recovery standard of 95%-115%.
To learn more about UV recovery, read our technote which compares the vial recovery of other major suppliers of recombinant proteins.
SDS-PAGE and densitometry analysis
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a method that separates proteins by mass. SDS is an ionic detergent that denatures and binds to proteins to make them uniformly negatively charged. This means that when an electronic current is applied to a polyacrylamide gel, the SDS-bound proteins will migrate down the gel towards the positively charged electrode, separated by size alone.
Proteins can be reduced before being run on an SDS-PAGE gel. In reducing conditions β-mercaptoethanol (β-ME or 2-ME) or dithiothreitol (DTT) is added; this reduces the disulfide bridges in proteins so that when they are run on the gel, they are better separated by size. Many of our growth factors are disulfide-linked dimers so running non-reduced proteins on the same gel as reduced proteins allows confirmation of the correct dimeric state.
As standard, we run all 3ug and 7ug of our recombinant proteins in reduced and non-reduced conditions to ensure there are no contaminants, aggregates or degradants. We also test our recombinant proteins by quantitative densitometry to ensure all our proteins are >98% pure.
Why are your proteins stated 98% pure, what is the other 2%?
We believe our proteins are >99.9% pure. However, due to limitations of gel staining and densitometry, there is an inherent error of +2%, which means that we can only ever state the limit of the assay, or >98% purity. To ensure purity, we always run a gel with a large amount of protein (7 ug) to ensure that we can detect any other proteins, aggregates, or degradation products. The SDS-PAGE results are validated with mass spectrometry analysis for every lot of protein manufactured.
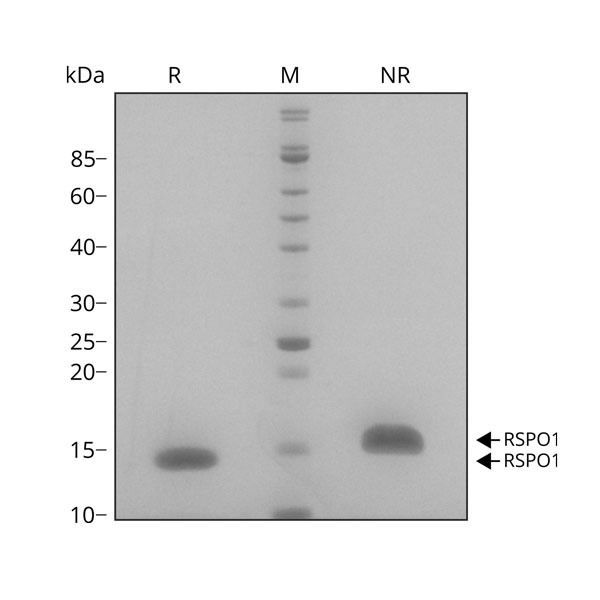
Example result
Bioactivity assay
It is very hard to determine a single value for the activity of a growth factor as growth factors generally have a large range of potential effects. A luciferase reporter assay is used to quantify the response of transfected cells to a growth factor by assaying the expression of luciferase (the reporter gene). As the biological activity of a growth factor is measured by its effect on a defined cell type in standardized conditions, it may not accurately represent your own culture conditions, however, it will provide confirmation that the growth factor is able to elicit a biological response.
To determine this bioactivity, a vector containing a firefly luciferase gene under the control of a growth factor-appropriate regulatory region is introduced into cells, such as HEK293Ts. These are then incubated with a dilution series of the growth factor. After a defined incubation period, the bioactivity is measured by quantifying the enzymatic activity of luciferase. This involves lysing the cells and adding the luciferase substrate, luciferin. The amount of light emitted from the reaction is directly proportional to the amount of luciferase in the sample and consequently can be used as a measure of the level of response the growth factor imparts on the reporter gene. In our QC experiments, we also quantify Renilla luciferase which is expressed constitutively by co-transfecting the same cells with a vector containing a Renilla luciferase gene. This acts as a normalization control for transfection efficiency and the cell number in each well. The growth factor response can then be expressed as a firefly/Renilla ratio. Plotting concentration vs. F/R ratio allows an EC50 (half-maximal effective concentration) to be calculated.
The bioactivity of some of our growth factors is measured using a proliferation assay. The ability of the growth factor to stimulate the proliferation of a relevant cell type is measured through a luminescence method related to the number of live cells (amount of ATP) after a defined number of days in culture. Again, plotting growth factor concentration vs. luciferase units allows an EC50 to be calculated.
The EC50 is the concentration of the growth factor which gives 50% of the maximum effect on the given cell population.
When shopping for growth factors, you want to confirm that the protein has been established to be consistently bioactive across multiple lots, ensuring each lot you receive will be equally as active as that reported. Our quality control process includes validation with a previous lot to ensure excellent lot-to-lot consistency (e.g. Figure 1). This consistency is essential when treating cells with growth factors to prevent experiment variability. The activity of a growth factor can differ significantly depending on experimental conditions. This means that comparisons between growth factors from different suppliers are tricky to make. At Qkine, we have validated most of our growth factors against other suppliers’ proteins under our experimental conditions to ensure that we are providing you with products that are as good as, or better than, those you may have used previously.
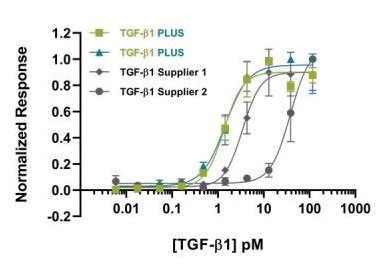
Human-TGFb1-PLUS
Qk010 protein bioactivity lot #104273
Mycoplasma testing
Mycoplasmas represent a group of microorganisms that are characterized by their lack of a rigid cell wall. They are one of the smallest self-replicating organisms known and can subsequently pass through anti-bacteriological filters. They represent a major challenge in cell culture laboratories as they can produce a variety of effects on cells, are resistant to most antibiotics and can impact the reliability and reproducibility of experimental results. For this reason, it is crucial that all reagents purchased for stem cell culture have been tested for the absence of mycoplasma.
All Qkine proteins are tested for the presence of mycoplasma in two assays. The first is a selective biochemical test that exploits the activity of mycoplasmal enzymes which are found in the vast majority of about 200 mycoplasma species but are not present in eukaryotic cells. The bioluminescence is measured and can detect contamination from Mycoplasma, Acholeplasma, Entomoplasma and Spiroplasma. We also use a PCR-based method to amplify the conserved 16S ribosomal RNA coding region within mycoplasma genomes, thereby providing an extensive, highly sensitive, and efficient detection method. This test detects three genera of Mycoplasmatales – Mycoplasma, Acholeplasma, and Ureaplasma.
Sterility testing
To ensure no contamination from bacteria or fungi, we carry out sterility testing in anaerobic and aerobic conditions through direct inoculation of fluid thioglycolate medium (FTM) and tryptic soy broth (TSB) and a 14 day incubation protocol followed by turbidity testing. This test is based on US Pharmacopoeia 71, EU Pharmacopoeia 2.6.1, and JP Pharmacopoeia 4.06 requirements. Positive controls of Pseudomonas aeruginosa, Escherichia coli, Candida albicans, Aspergillus brasilensis, Bacillus spizizenii and Staphylococcus aureus are included alongside each test.
Highly bioactive & animal-free recombinant proteins
We are confident that the quality and bioactivity of our recombinant proteins will ensure that you achieve your research goals. Our dedicated team of stem cell specialists is available to answer any queries you may have and to give expert support when required.