Recombinant bovine/porcine FGF-2 (145 aa) protein (Qk040-FG)
Recombinant bovine/porcine FGF-2 (145 aa) protein (Qk040-FG)Recombinant bovine/porcine FGF-2 (145 aa) protein (Qk040-FG)
Price range: £135.00 through £670.00
Recombinant bovine/porcine FGF-2 protein 145 aa (bFGF/basic FGF) for the development of species-specific bovine (cow) and porcine (pig) cellular agriculture protocols and veterinary research applications. Can be used in comparative cell culture media optimization studies alongside Qk056-FG, the 154 aa form of porcine/bovine FGF-2.
Qkine high qualify food recombinant bovine/porcine FGF-2 protein 145 aa protein is a high purity 16 kDa protein, animal origin-free (AOF) and carrier-protein free (CF).
In stock
Orders are typically shipped same or next day (except Friday).
Easy world-wide ordering, direct or through our distributors.
Price range: £135.00 through £670.00
Buy online with secure credit card or purchase order. For any questions, please email orders@qkine.com
Summary:
- High purity recombinant bovine/porcine FGF-2 protein 145 aa / bFGF (Uniprot: P03969)
- 16 kDa
High quality food grade recombinant protein
>98%, by SDS-PAGE quantitative densitometry
Animal origin-free (AOF) and carrier protein-free
Expressed in E. coli
Manufactured in the UK under a food manufacturing HACCP regime
Lyophilized from Tris, NaCl, CyS, mannitol
- Resuspend in sterile-filtered water at >50 µg/ml, add carrier protein if desired, prepare single use aliquots and store frozen at -20 °C (short-term) or -80 °C (long-term).
Featured applications:
Bovine and porcine stem cell expansion and maintenance
Bovine and porcine primary cell culture
Culture of porcine (pig) muscle stem cells
Culture of bovine (cow) muscle stem cells
Cellular agriculture and cultivated meat cell culture media optimization
Cellular agriculture process development
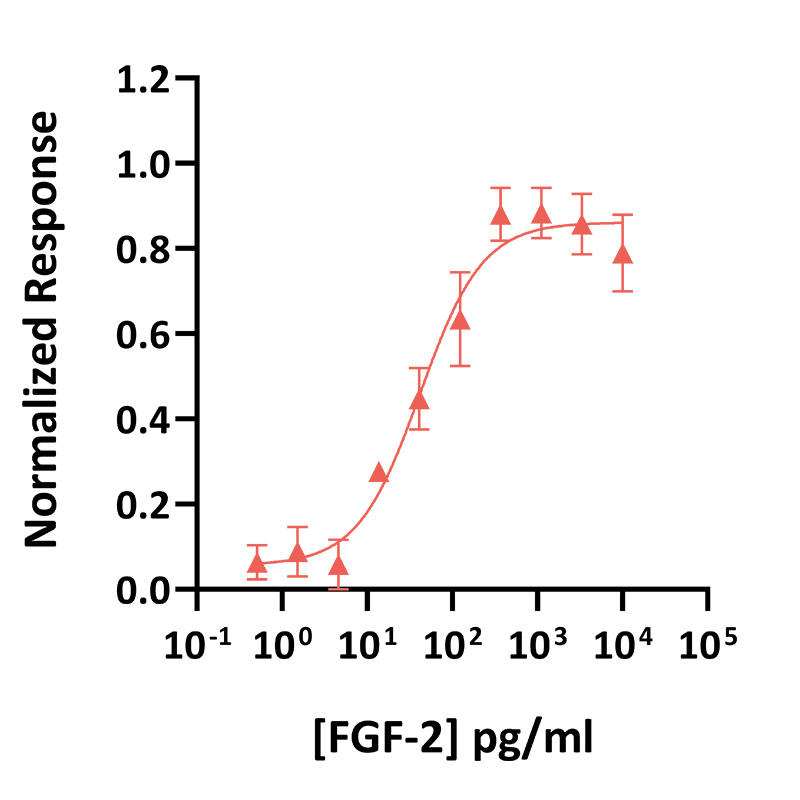
FGF-2 activity is determined using the Promega serum response element luciferase reporter assay (*) in transfected HEK293T cells. EC50 = 160 pg/ml (10 pM). Cells are treated in triplicate with a serial dilution of FGF-2 for 3 hours. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. Data from Qk040 lot #104301. *Promega pGL4.33[luc2P/SRE/Hygro] #E1340
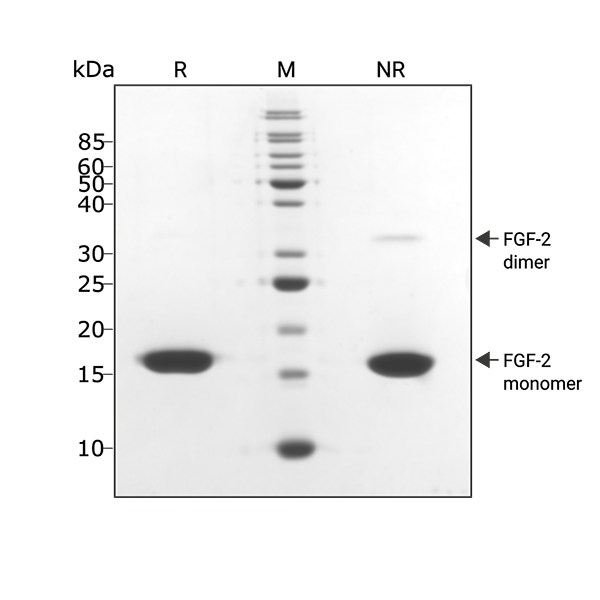
Recombinant bovine/porcine FGF-2 protein 145aa migrates as a single major band at 16 kDa in non-reducing (NR) conditions and some dimeric protein migrating at 32 kDa. Upon reduction (R), only the 16 kDa band is visible. No contaminating protein bands are visible. Purified recombinant protein (3 µg) was resolved using 15% w/v SDS-PAGE in reduced (+β-mercaptothanol, R) and non-reduced (NR) conditions and stained with Coomassie Brilliant Blue R250. Data from Qk040 batch #104301.
Further quality assays
Mass spectrometry: single species with expected mass
Recovery from stock vial: >95%
Endotoxin: <0.005 EU/μg protein (below level of detection)
Full raw materials traceability, allergen analysis, CoO, CoA, beta-lactam-free and animal origin-free certification available
We are a company founded and run by scientists to provide a service and support innovation in stem cell biology and regenerative medicine. All our products are exceptionally high purity, with complete characterisation and bioactivity analysis on every lot.
Protein background
Fibroblast growth factors (FGFs) are a family of growth factors which regulate a wide range of essential biological functions including cell proliferation and survival, migration and differentiation [1]. The human FGF family has 18-22 members grouped into 6-7 subfamilies based on sequence homology and phylogeny [2]. The FGF family of growth factors have critical roles during both vertebrate and invertebrate embryonic development, in adult cells they modulate tissue maintenance, wound healing and angiogenesis [1,2]. The FGF ligands bind to 4 receptors (FGFR1-4), transmembrane receptors with intracellular tyrosine kinase activity [2, 3]. Once activated FGFRs recruit Src homology-2 (SH2) or phosphotyrosine binding (PTB) domain-containing signaling proteins leading to activation of intracellular signaling pathways [1]. The main signaling pathways activated by FGF binding are the RAS/MAP kinase pathway, PI3 kinase/Akt pathway, and PLCγ pathways [1-3].
Fibroblast growth factor 2 (FGF-2) also known as basic fibroblast growth factor (bFGF) has a broad range of physiological roles including regulation of cell growth, survival and proliferation. FGF-2 is one of the FGFs which binds to and signals through all four of the FGFRs [4]. FGF-2 is an essential growth factor for the maintenance of pluripotency in human embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) [4-6] and mesenchymal stem cells (MSCs) [7]. FGF-2 preserves pluripotency through interaction with PI3 kinase/Akt, ERK1/2, JAK/STAT and PLCγ pathways by activation of activin A. In feeder culture systems FGF-2 stimulates production of essential growth factors and cytokines from the feeder layer [7].
FGF-2 is an important factor in muscle development and healing after injury [8]. Cultured meat is a potential sustainable food generated through the in vitro myogenesis of muscle satellite (stem) cells (MSCs), involving MSC culture and differentiation, and mature muscle cell processing for flavor and texture [9]. FGF-2 induces the proliferation of cultured myoblasts and inhibits their differentiation [8, 9].
FGF-2 is an important component of Beefy9, a serum free media developed for the in vitro expansion of MSCs for cultured meat, Beefy9 allows for expansion of MSC while preserving myogenicity [10]. The two forms of recombinant bovine/porcine FGF2 protein 154 aa (Qk056-FG) and 145 aa (Qk040-FG) available from Qkine facilitate the development of fully optimized serum-free culture media for cellular agriculture applications.
FAQ
Fibroblast growth factor 2 (FGF-2), also known as basic fibroblast growth factor (bFGF) is a growth factor and signaling protein.
FGF-2 is expressed in a developmental and tissue specific manner. It’s expression is tightly controlled in normal tissues and it can be detected in all major tissues.
FGF-2 is essential for normal embryonic development. It has roles in cell survival and proliferation, angiogenesis, tumorigenesis, wound healing and tissue repair.
FGF-2 binds to and signals though all four of the FGF receptors FGFR1-4.
FGFRs phosphorylate specific tyrosine residues and activate the RAS-MAPK, PI3K-AKT, PLCγ, and STAT intracellular signaling pathways.
FGF-2 is used to maintain the pluripotency of stem cells in culture.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.
For use in manufacturing of cellular or gene therapy products. Not intended for in vivo applications.

Receive an Amazon gift voucher when you leave us a review.
£25, $30 or €30 for reviews with an image and £10, $15 or €15 for reviews without an image