Cell therapy grade growth factors and cytokines
Qkine cell therapy grade high purity animal origin-free proteins are manufactured as GMP grade equivalents in an ISO 9001:2015-certified facility, under ISO 20399:2022 standards with GMP compliance and defined quality criteria and documentation.
Cell therapy grade growth factors and cytokines
Cell and gene therapies offer the potential to treat a wide range of diseases, many of which have previously been difficult or impossible to treat effectively.
Cell therapy involves the transplantation of cells with the intention to cure or prevent a disease and gene therapy the introduction of genes to replace missing or defective ones. New cell therapies are being developed and tested all the time, often requiring the ex vivo expansion of cells in protocols requiring materials such as growth factors and cytokines produced under Good Manufacturing Practice (GMP). This system of regulations, guidelines, and best practices ensures products, especially drugs, biologics (like cell and gene therapies), and medical devices are consistently produced and controlled to high quality standards.
Qkine have launched a range of our high purity animal free growth factors and cytokines as cell therapy grade with additional quality testing as a high quality value equivalent to other suppliers GMP grade recombinant protein offering. As GMP is not a 'grade' but a collection of guidelines, labeling ‘GMP grade’ can be ambiguous, so we have developed our 'cell therapy grade' range to have clearly defined quality assurance and documentation making it suitable for cell and gene therapy development.
All Qkine cell therapy grade proteins are manufactured in our ISO 9001:2015-certified facility, under ISO 20399:2022 standards with GMP compliance, defined quality criteria and documentation.

Interested in cell therapy grade proteins?
Contact us to keep updated with our cell therapy grade proteins.
Qkine are manufacturing our range of cell therapy grade proteins workflow by workflow, but if there is something else you need please let us know. All our research use proteins are potentially available as cell therapy grade, contact us for details and lead time.
Growth factors and cytokines for cell and gene therapy
Hematopoietic and immune cell therapy
Immune cell therapy usually requires the ex vivo expansion of patient or donor immune cells to aid in cancer cell killing. Healthy hematopoietic stem cells can also be used to replace diseased bone marrow derived immune cells.
Pluripotent stem cell therapy
Pluripotent stem cell therapies can be developed from embryonic or induced pluripotent cells. These therapies can be developed to treat a wide range of currently incurable diseases such as degenerative eye diseases or diabetes.
Neural stem cell regenerative therapy
Neural stem cell therapy has the potential to treat neurological disorders by replacing lost neurons, promoting nerve regeneration, and releasing neurotrophic factors that reduce inflammation and improve the neural environment.
Cell therapy grade TGF-β1 PLUS™
Our uniquely animal origin-free TGF-β1 PLUS™ is now available as cell therapy grade. TGF-β1 can be used in the maintenance of pluripotent stem cells for therapeutic use and induction of regulatory T cells (iTregs).
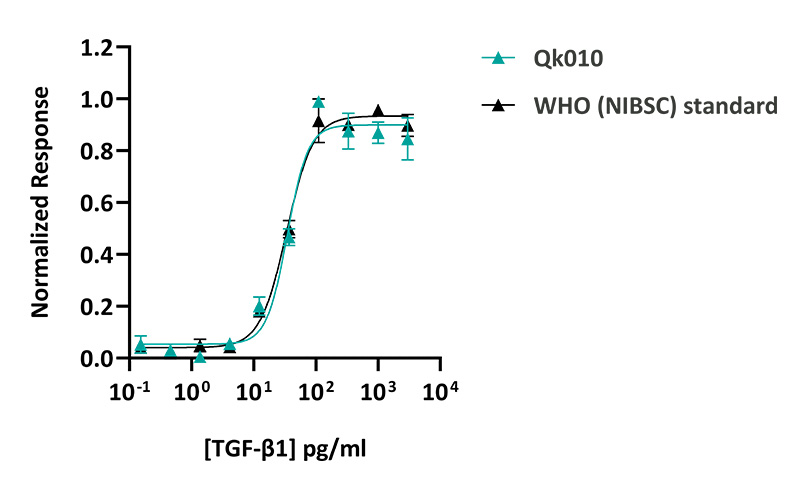
Qkine TGF-β1 PLUS™ was as bioactive as the WHO (NIBSC) standard TGF-β1. TGF-β1 PLUS™ activity was determined using a CAGA luciferase reporter assay in transiently transfected HEK293 cells. Transfected cells were treated in triplicate with a serial dilution of TGF-β1 PLUS™ for 6 hours. Firefly activity was measured and normalized to the control Renilla luciferase activity. Qk010 #204560 EC50 = 35 pg/ml (1.46 pM), WHO (NIBSC) EC50 = 34 pg/ml.
Cell therapy grade IL-15 protein
Recombinant IL-15 stimulates the proliferation and activation of multiple T cell subsets including NK, NKT, Th17, Treg, and CD8+ memory cells. Cell therapy grade IL-15 can be used for the preconditioning of CAR T cells.
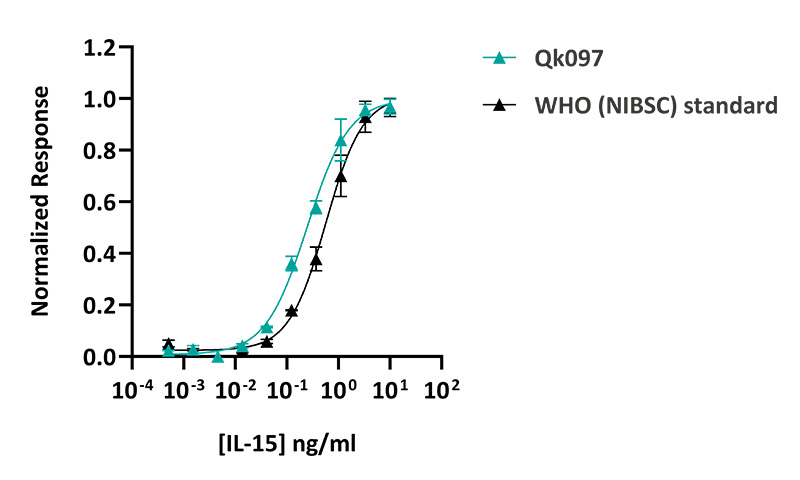
Qkine IL-15 was more bioactive than the WHO (NIBSC) standard IL-15. IL-15 activity was determined using a SRE luciferase reporter assay in transiently transfected HEK293 cells co-transfected with the IL-15 receptors (IL-2Rβ and IL-2Rγ). Transfected cells were treated in triplicate with a serial dilution of IL-15 for 3 hours. Firefly activity was measured and normalized to the control Renilla luciferase activity. Qk095 #204736 EC50 = 0.25 ng/ml (19.4 pM). WHO (NIBSC) standard EC50 = 0.57 ng/ml.

Why are Qkine’s new products labelled cell therapy grade and not GMP grade?
Why are the products labelled cell therapy grade and not GMP grade? Although GMP-grade is a recognized term within the growth factor space, it is not an accreditation that can be strictly applied to products outside of the pharmaceutical, cosmetic or food industries, read our blog to find out more.

Animal origin-free proteins for cell therapy
Qkine cell therapy grade proteins are animal origin-free, but why is this so important? The FDA are clear in their recommendation that animal origin-free reagents are used for increased safety in cell and gene therapy manufacture, read our blog to find out more.