Introduction
Neurodegenerative diseases involve the degeneration of neurons and encompass a range of conditions including age-related cognitive disorders (Alzheimer’s and Parkinson’s diseases), amyotrophic lateral sclerosis (ALS), and spinal muscular atrophy (SMA). Currently, there are no definitive treatments and studying neurodegenerative diseases is challenged by the intricate nature of neural tissue and the limited availability of suitable model systems. Advancements in human induced pluripotent stem cells (iPSCs) have provided novel sources of valuable in vitro model systems to study neural development, disease mechanisms, and drug treatment. Through specific exposure to small molecules, including growth factors and cytokines, iPSCs can be directed to differentiate into neural stem cells and subsequently into various neural and glial cell types.
Brain-Derived Neurotrophic Factor (BDNF) and Glial Cell-Derived Neurotrophic Factor (GDNF) are part of the neurotrophic factor family that play a crucial role during embryonic development and the maintenance of the nervous system during adulthood [1]. In cell culture, they are essential growth factors in the differentiation of iPSCs into motor neurons (MNs) and maintenance of the distinctive characteristics and functions of cultured neurons over prolonged durations [2 , 3]. Therefore, it is essential to have high-quality and bioactive proteins to generate reproducible, reliable, and physiologically relevant neural cultures. This collaborative study compares BDNF and GDNF from Qkine and R&D Systems in their ability to support the differentiation and maintenance of iPSC-derived motor neurons.
Materials and Methods
Differentiation of iPSCs to MNs
Human iPSCs were cultured in mTeSR Plus Media (Stem Cell Technologies, 100-0276) supplemented with 1% Penstrep (Thermofisher Scientific, 15140122). iPSCs were passaged using 5 mM EDTA in 1x PBS onto Matrigel (Corning, 354234) coated plates. Motor Neurons were generated using using a well-established protocol [4,5]. Spheres, or embryoid bodies (EBs), were generated from detached and small aggerates of iPSCs resuspended in mTeSR Plus supplemented with 10 µM Y-27632 (MedChemExpress, HY-10583) and 10 ng/ml FGF-2 (Millipore, 3216002). Suspension was cultured in ultra-low attachment 10cm plates (Corning, 4615) on an orbital shaker in incubator set to 37˚C and the resulting EBs were differentiated using the protocol displayed below (Figure 1). To compare the efficacy of Qkine vs. R&D Systems BDNF and GDNF (Table 1), each compound set was used to differentiate and culture independent sets of EBs and the resulting MNs.

Figure 1: Differentiation Protocol for iPSCs into MNs. Schematic describing media formations used during the differentiation of iPSCs into MNs.
| Provider | Catalogue Number | Expression System | |
|---|---|---|---|
| Recombinant Human BDNF | Qkine | Qk050 | Escherichia Coli |
| R&D Systems | 248-BDB | Spodoptera frugiperda (baculovirus) | |
| Recombinant Human GDNF | Qkine | Qk051 | Escherichia Coli |
| R&D Systems | 212-GD | Mouse myeloma cell line |
Table 1: Origins of the recombinant human BDNF and GDNF proteins used in this study.
Plating and Culturing of hiPSC-derived MNs
Following differentiation EBs were dissociated to single cells using the Worthington papain dissociation system (Worthington; 9035-81-1, 9048-46-8, 9003-989, 9001-73-4). MNs were plated at a density of 80,000 cells/well into black flat-bottom Greiner µClear 96-well plates (Greiner Bio-One, 655090) coated with 50 µg/ml PolyD-Lysine (Sigma-Aldrich, A-003-E), 1x Borate Buffer (Thermofisher Scientific, 28341), and 2.5 µg/ml of Laminin (Thermofisher Scientific, 23017015). Media was changed every 2-3 days following plating. On days 2 (DIV2) and 8 (DIV8), individual plates of MNs were fixed with 4% methanol-free PFA (Pierce, 28908) for 12-15 min. Following fixation cells were washed 3x with 1x Dulbecco’s PBS -Ca/-Mg (Thermofisher Scientific; 14190169).
Immunocytochemistry, Imaging, and Analysis of iPSC derived MNs
Immunocytochemistry for the dendritic marker MAP2 (Novus Biologicals; NB300-213) and MN marker Islet1 (Abcam; Ab109517) was carried out in 0.25% Triton and 1.0% Normal Goat Serum in 1x Dulbecco’s PBS -Ca/Mg. Secondary antibodies used were anti-chicken IgG (H+L) Alexa Fluor 647 (A21449) and anti-rabbit IgG (H+L) Alexa Fluor 546 (A11035). Nuclei were stained using Hoechst 33342 (Invitrogen; H3570). Stained MNs were then imaged using the Perkin Elmer Operetta CLS’s 40x water objective in non-confocal mode, and the images were quantified using Perkin Elmer’s Columbus browser supported software. Brightfield images of EBs and MNs were taken using a Nikon Eclipse Ts2 microscope with 4x and 20x objectives, respectively.
Results
Recombinant BDNF and GDNF proteins successfully differentiated human iPSC into MNs
Recombinant BDNF and GDNF proteins from both Qkine and R&D systems were effective at supporting the formation of end-stage differentiated EBs. EBs were spherical with a smooth surface and had a ~500-550 µM diameter by day 15 (EB15, figure 2). MNs dissociated from these EBs were cultured in the same media supplemented with recombinant BDNF and GDNF proteins from Qkine or R&D systems. After 2 days (DIV2), the motor neurons displayed healthy neuronal morphology with clear neurite extension. By day 5 (DIV5), the neurons developed increased arborization and some clustering which is typical for aging iPSC derived neuronal cultures (Figure 2).
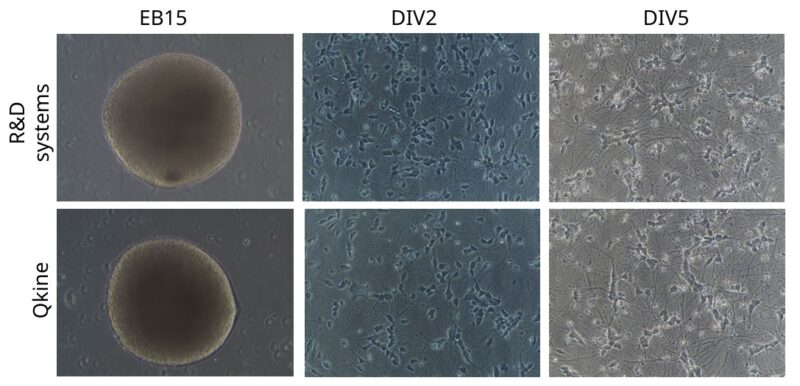
Figure 2: Morphological and viability assessment of embryoid bodies and dissociated motor neurons cultured with BDNF and GDNF. Brightfield images representing EBs at day 15 (EB15) of differentiation protocol (scale bar = 250 µm) and dissociated MNs from the same EBs after 2 (DIV2) and 5 (DIV5) days in vitro (scale bar = 100 µm).
MNs Expressed MAP2 and Islet 1
Immunocytochemistry was performed to confirm that the cells produced were MNs using the presence of neuron-specific dendritic marker, Microtubule associated protein 2 (MAP2), and the characteristic motor neuron marker, Islet1, at different timepoints (DIV2 and DIV8) (Figure 3A). Both proteins from Qkine and R&D systems resulted in highly pure neuronal cultures as demonstrated by 95% of cells being MAP2+ at the two tested timepoints (Figure 3B). 40-50% of the culture exhibited the characteristic motor neuron marker Islet1 at DIV2 and DIV8 for both suppliers, indicating that MAP2+/Islet1+ motor neurons were reliably maintained in culture (Figure 3C). Additionally, both Qkine and R&D systems show similar maintenance and survival of the MAP2+/ Islet1+ MNs in culture from DIV2 to DIV8 (Figure 3D).
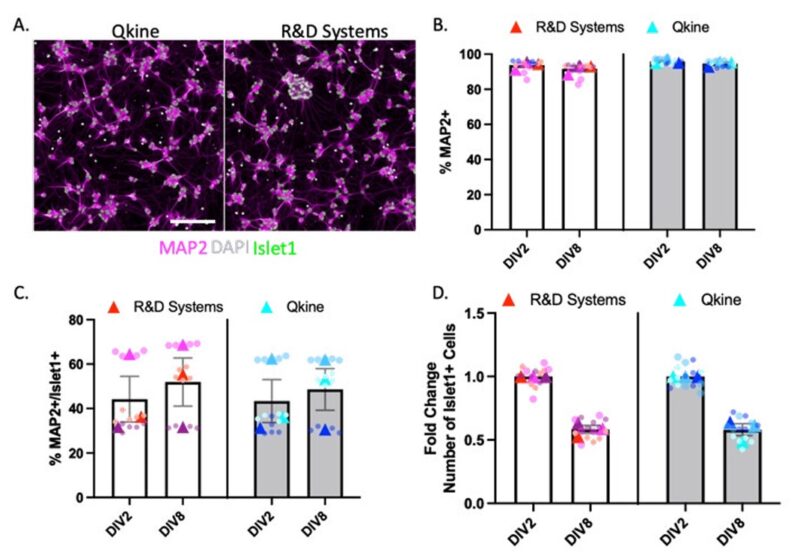
Figure 3: Characterisation of iPSC-derived MNs. Immunocytochemistry images representing iPSC-derived motor neurons stained with DAPI (grey), MAP2 (magenta), and Islet1 (green) at DIV8 (A, scale Bar = 50 µm). Quantification of the percentage of MAP2+ neurons (B), percentage of MAP2+/Islet1+ cells (C), fold change of the number of Islet1+ cells after DIV2 and DIV8 (D).
Discussion
Recombinant human BDNF and GDNF proteins from both Qkine and R&D systems effectively generated and maintained healthy iPSC-derived MNs in both 2D and 3D culture systems. MNs generated with growth factors from both suppliers expressed the markers MAP2 and Islet1 in a similar manner from DIV2 to DIV8. Qkine BDNF and GDNF are reliable and animal origin-free (AOF) proteins for differentiation of neural cultures and their subsequent maintenance. AOF proteins reduce variability from animal derived materials and ensure a more controlled environment and reproducibility in the differentiation of MNs. With the increasing use of iPSC derived neuronal models, including human iPSC derived MNs, the ability to produce these neurons on a more affordable scale becomes essential. AOF proteins meet the scale-up challenges of culturing MNs as they can be produced in bulk, whilst remaining highly pure and bioactive. In addition, integrating AOF recombinant proteins into the culture of motor neurons ensures that these cultures can be translated to downstream clinical applications if required.
High-quality growth factors are essential for developing and maintaining robust, reproducible, and physiologically relevant neural cultures. Growth factors developed in an animal-free expression system have a higher lot-to-lot consistency and fewer contamination risks from viruses, prions, and other animal-derived ingredients. All growth factors and cytokines from Qkine pass stringent biochemical and bioactivity quality control testing, ensuring you complete confidence in the reagents for your neural stem cell cultures.
References
1.Skaper, S. D. The Neurotrophin Family of Neurotrophic Factors: An Overview. in Neurotrophic Factors: Methods and Protocols (ed. Skaper, S. D.) 1–12 (Humana Press, 2012).
2.Huang, E. J. & Reichardt, L. F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 24, 677–736 (2001). doi: 10.1146/annurev.neuro.24.1.677
3.Wichterle, H., Lieberam, I., Porter, J. A. & Jessell, T. M. Directed Differentiation of Embryonic Stem Cells into Motor Neurons. Cell 110, 385–397 (2002). DOI: 10.1016/s0092-8674(02)00835-8
4. Rigamonti, A., Repetti, G.G., Sun, C., et al. (2016). Large-scale production of mature neurons from human pluripotent stem cells in a three dimensional suspension culture system. Stem Cell Reports 6, 993–1008. DOI: 10.1016/j.stemcr.2016.05.010
5. Rodriguez-Muela N, Litterman NK, Norabuena EM, et al. Single-Cell Analysis of SMN Reveals Its Broader Role in Neuromuscular Disease. Cell Rep. 2017 Feb 7;18(6):1484-1498. doi:10.1016/j.celrep.2017.01.035. DOI: 10.1016/j.celrep.2017.01.035