Protein tags, why and why not?
Recombinant proteins can be ‘tagged’ by adding a (usually) short amino acid sequence that is not naturally occurring and can therefore be used to purify the protein from a complex mixture more easily. Other frequently used protein tags allow later protein modification such as biotinylation, coupling to other proteins for subsequent biochemical protocols or improve folding or yield during protein expression.
To improve the reproducibility and reliability of experiments relying on recombinant proteins, including biochemical assays and cell culture where these proteins are often critical components of cell culture media, it is important to use tag-free proteins unless the application specifically requires a tagged form of the protein.
What can protein tags be useful for?
Protein affinity tags
Affinity tags can be added to recombinant proteins to make them easier to purify. The most commonly used affinity tag is the polyhistidine or ‘His’ tag. The His tag sequence consists of 4-10, usually 6, histidine amino acids and can be added through simple cloning techniques. Affinity purification with a His tag uses the interaction of the basic imidazole ring of histidine with divalent metal ions, such as nickel or cobalt [1].
Glutathione S transferase (GST) is a larger protein tag at 26 kDa, which can be used for purification mediated by its high affinity interaction with glutathione [1]. GST, along with some other protein tags, can also act as a protein chaperone, promoting correct folding and preventing aggregation in inclusion bodies during expression in E.coli [2]. Other protein tags, such as myc tag can be purified by binding to an antibody, a summary of some commonly used affinity purification tags can be found in table 1.
| Tag | Amino acid sequence | Mwt (kDa) | Affinity |
|---|---|---|---|
| His | HHHHHH | 0.8 | Divalent metal ions |
| GST | MSPILGYWKIKGLVQPTRLLLEYLEEKYEE HLYERDEGDKWRNKKFELGLEFPNLPYYID GDVKLTQSMAIIRYIADKHNMLGGCPKERA EISMLEGAVLDIRYGVSRIAYSKDFETLKV DFLSKLPEMLKMFEDRLCHKTYLNGDHVTH PDFMLYDALDVVLYMDPMCLDAFPKLVCFK KRIEAIPQIDKYLKSSKYIAWPLQGWQATF GGGDHPPKSD | 26 | Glutathione |
| Myc | EQKLISEEDL | 1.2 | Antibody |
| Flag | DYKDDDDK | 1.0 | Antibody |
| ALFA | SRLEEELRRRLTE | 15 | Antibody |
| HA | YPYDVPDYA | 1.1 | Antibody |
| V5 | GKPIPNPLLGLDST | 1.4 | Antibody |
Table 1. Commonly used peptide tags for affinity purification.
Other uses for protein tags
Protein tags can have uses other than for direct affinity purification. The Avi-tag (GLNDIFEAQKIEWHE) can be used for consistent biotinylation of proteins allowing high affinity binding to streptavidin and avidin. Biotinylation is then useful for affinity chromatography and detection methods, such as ELISA, SPR and immunofluorescence and has important uses in drug and target discovery. Other protein tags can be used for protein localization studies, such as green fluorescent protein (GFP) which is naturally fluorescent.
What problems can protein tags cause?
Most protein tags are small and therefore often thought to have little or no effect on proteins, however a scan of the literature finds several cases where tags have been shown to alter protein behavior, sometimes with serious consequences. Unfortunately, it is difficult to predict the impact of even a small protein tag on the functionality of the protein. In a clinical or indeed cellular agriculture (cultivated meat, fish, fat and dairy) cell manufacturing context, the presence of tags may have more severe consequences by introducing non-natural amino acid sequences that may be allergenic and are therefore subject to additional scrutiny by regulators.
Protein tag interference with bioactivity
A His-tag may interfere with a protein’s ability to bind ligands, cause the protein to aggregate, or change its solubility [3]. This could affect substrate specificity, kinetic constants, or protein dynamics. For example, Lacy and Sanderson (2002) found evidence that a 6×His tag increased the heparan sulfate-binding capability of a recombinant protein called Sp17, increasing the protein’s ability to promote cell-cell adhesion [4]. As heparan sulfate is ubiquitously expressed in extracellular matrices and on cells, if a protein is to be tested in vivo and in vitro with intact cells, then using a His-tag may not be appropriate.
Qkine proteins are all produced free of protein tags, however, during custom production of an Avi-tag activin E PLUS, we found that there was a reduction in bioactivity relative to the untagged version, increasing the EC50 10-fold (figure 1), providing additional direct experimental evidence for changes in protein activity due to the presence of a small N-terminal protein tag.
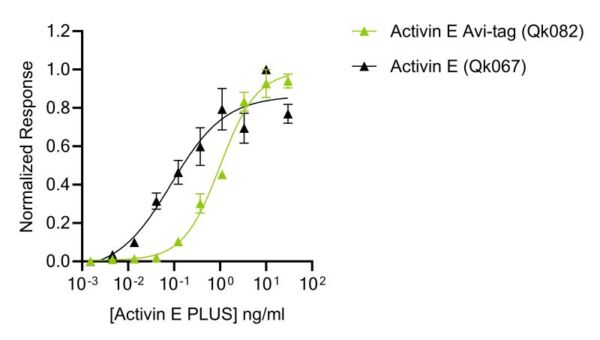
Figure 1. Bioactivity of Avi-tag activin E PLUS compared to untagged activin E PLUS. Recombinant activin E PLUS activity was determined using a Smad reporter assay in HEK293 cells co-transfected with the activin E co-receptor ALK7. Cells were treated in triplicate with a serial dilution of activin E PLUS (Qk067) or Avi-tag activin E PLUS (Qk082) for 6 hours. Firefly activity was measured and normalized to the control Renilla luciferase activity.
Addition of an N-terminal Avi-tag to activin E PLUS increased the EC50 approximately 10-fold, from 0.09 ng/ml to 1 ng/ml.
Recombinant protein yield and purity
Purification tags can be removed with a site-specific protease, but in practice, this almost always leaves at least one foreign residue behind, and introducing a protease step can reduce yield. For example, Leite et al. (2021) found that that the production of NRG-1 required the 6xHis tag to be cleaved before use, reducing the yield available for their stem cell culture media [5].
Another major drawback of His-tag affinity purification of proteins expressed in E. coli is the co-purification of naturally occurring histidine-rich host proteins that contaminate the recombinant protein of interest. The most effective means of increasing the purity of a target protein is to use additional affinity tags or multiple purification steps; however, this lowers the yield and increases the purification time and cost [6].
As well as potential problems caused by the tag itself, IMAC resin can also have adverse effects on proteins due to the co-elution of metalloproteinases and oxidation of eluted proteins due to leached metal ions [7]. The cost of using these resins at scale, and lack of compatibility with standard bioprocesses also makes these unsuitable for scale-up of recombinant protein expression.
Protein tags in therapeutics
If proteins are to be used as therapeutics, then tags must be cleaved off, and assays performed that show the tag and the protease are not present in the final product. Any remaining residues on the protein will need to be shown to not interfere with biological activity or lead to neoallergen formation. In addition, there are considerations of scalability, affinity purification is unlikely to be the safest, cleanest, most scalable, and cost effective way of making a specific protein for clinical or cell therapy manufacturing use
Protein expression and purification without tags
As part of our commitment to providing the highest quality proteins for our customers, we purify our proteins using a combination of different techniques that don’t require external tags. In the few cases where protein tags are used within our manufacture process we cleave off the tag and extensively purify the protein using the methods below. Our quality control assays confirm the full removal of the tag from the final recombinant protein.
Routinely, we separate proteins using their intrinsic properties. Each protein has different properties, so our experienced R&D team chooses the appropriate techniques based on our knowledge of the protein. We then design and optimize protocols to ensure each batch produces pure, bioactive protein.
An example of a technique we use is cation-exchange chromatography, in which proteins are separated by overall charge. We know the amino acid sequence of the target protein, so we use buffer conditions in which the protein has a net positive charge. The target protein will bind to a negatively charged resin, and the strength of the binding will depend on its charge. Proteins can be separated by eluting with increasing salt concentrations, as the salt concentration required for elution depends on the charge of the protein.
Summary
Qkine growth factors are manufactured to the highest quality standards and are tag free, carrier protein free and free from animal-derived contaminants, delivering low endotoxicity and high purity. All our proteins conform to our Nine-point Qkine Quality Commitment.
At Qkine, we are committed to raising the standards of growth factors, cytokines and related proteins to better support long-term and complex cell cultures. We are a science-led team, please reach out with any questions or requests to support@qkine.com.




