At Qkine, we have repeatedly highlighted the need for stricter process and quality control in the manufacture of recombinant growth factors to prevent cross-contamination with related growth factors. Contamination with other bioactive proteins leads to a lack of inter- and intra-lab reproducibility or, perhaps most concerningly, incorrect scientific conclusions.
Perhaps the most well-known published example of this is TGF-β1 contamination of GDF-15 due to the use of mammalian protein expression systems, this raises an important consideration for sourcing and qualifying reagents but ultimately has an understandable biological basis. However, extremely concerningly, the recent article from Svraka et al [1] highlights another challenge, cross-contamination due to process errors during manufacture, which may be due to a lack of stringency in standard manufacturing procedures – such as using purification columns for more than one protein – or human error. This example also highlights the need for scientifically rigorous lot-specific quality control that must include mass spectrometry analysis of proteins to detect low-level contaminants.
Contamination of mammalian-expressed proteins with low levels of TGF-β1
Previous publications have focused on the need for animal origin-free growth factor expression to prevent co-purification of trace amounts of similar growth factors from mammalian sources. In 2017, Olsen et al. [2] reported on TGF-β contamination of commercially available recombinant GDF-15. Unlike other TGF-β family growth factors GDF-15 does not stimulate SMAD2 but signals through SMAD1/5. However, in their experiments, they found that GDF-15 stimulated SMAD2 phosphorylation in THP-1 cells in a time and dose-dependent manner (figure 1A).


Figure 1. Activation of SMAD2 by TGF-β contaminated GDF-15. (A) Mammalian expressed GDF-15 (Peprotech) lead to phosphorylation of SMAD2 which was not observed in E. coli expressed GDF-15 (Abcam). (B) Phosphorylation of SMAD2 by mammalian expressed GDF-15 was prevented using TGF-β neutralizing antibodies. Data from Olsen et al, 2017 [1].
This unexpected cellular response was proven to be due to TGF-β contamination in the mammalian expressed recombinant GDF-15, which potently stimulated SMAD2 phosphorylation down to 10 pg/ml (420 femtomolar) [2]. They eliminated the SMAD2 phosphorylation by recombinant GDF-15 using alternative E. coli expressed uncontaminated GDF-15 and by neutralizing TGF-β in mammalian GDF-15 (figure 1B).
Contamination of microbially-expressed proteins due to failings in process or quality control
A recent publication by Svraka et al. has further highlighted the need not just for animal origin-free recombinant growth factor sources but also the need for improvements in manufacturer QC. They have reported on cross-contamination of IL-17F with IL-4, a cytokine unrelated to their study [1]. IL-17F is a cytokine of interest through its role in inflammatory skin disorders.


Figure 2. Cross contamination of E. coli expressed recombinant IL-17F with IL-4. (A) IL-4 was detected in Peprotech IL-17F but not in R&D systems IL-17F. (B) STAT6 activation occurred only in IL-4 contaminated IL-17F. Data from Svraka et al 2025 [2].
They found unexpected phosphorylation of STAT6 and upregulation of CCL26 and IL4R in dermal fibroblasts with IL-17F stimulation, indicating a possible novel signaling pathway. However, further experimentation determined the upregulation of these genes associated with IL-4 stimulation were due to IL-4 contamination of the recombinant IL-17F and IL-4 was detected through western blotting (figure 2A). Uncontaminated bacterially expressed IL-17F from another source did not activate STAT6 (figure 2B).
Stringent process control is essential to prevent cross-contamination of highly bioactive growth factors and cytokines
We can’t determine how the cross-contamination of IL-17F with IL-4 occurred at their supplier. However, at Qkine we are intensely aware of the critical importance of removing any possibility of cross-contamination in recombinant protein manufacturing processes, not least because the GDF-15 example highlighted above personally impacted a close collaborator of Qkine’s founder Professor Marko Hyvonen. Stringent process development and process control, alongside extensive quality control on every lot of protein are essential to prevent waste of end-users’ experimental time and budget.
We have seen evidence at Qkine of how easily cross-contamination can occur if standard laboratory practices are followed. In our R&D laboratory, the team were refining processes for GDF-15 manufacture. Despite thorough decontamination and extensive cleaning of a purification column that had previously been used for activin A, a related protein, when our scientists purified GDF-15 on this column we saw clear evidence of contamination of GDF-15 with activin A in a bioactivity assay (Figure 3). Activin A activates the SMAD 2/3 pathway with femtomolar EC50, but GDF-15 is an unusual TGF-β super family member and has been proven not to activate this pathway.

Figure 3. Qkine GDF-15 QC for SMAD2/3 (activin A/TGF-β) bioactivity. Bioactivity was determined using a SMAD2/3 luciferase reporter assay in HEK293T cells. Cells were treated (in triplicate) with a serial dilution of GDF-15 in the presence or absence of 10 µM of the activin A inhibitor follistatin. In this lot, contaminating activity was seen (green line), which was inhibited by follistatin (black line), confirming that contaminating activin A is causing this activity. Data from Qk017 lot #010 (not for sale).
As cross contamination in commercial sources of GDF-15 is such a common problem Qkine has built a bioassay into our standard lot-specific quality control procedure for this protein to ensure there is no activation of SMAD2/3 in our preparations (figure 4).
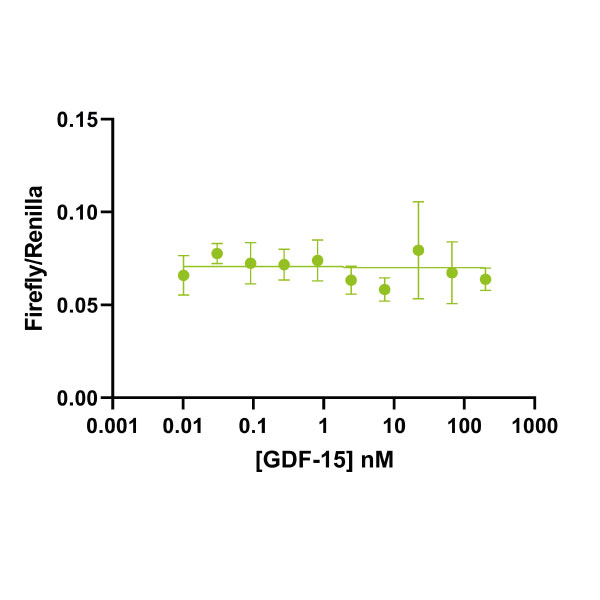
Figure 4. Qkine GDF-15 QC for SMAD2/3 activity. Bioactivity was determined using a SMAD2/3 luciferase reporter assay in HEK293T cells. Cells are treated (in triplicate) with a serial dilution of GDF-15 (Qk017) or TGF-β1 (Qk010) for 6 h. Firefly luciferase activity is measured and normalized to the control Renilla luciferase activity. Data from Qk017 lot #104282.
To ensure no cross-contamination of our high-purity growth factors, cytokines and other bioactive proteins at Qkine we have developed a Nine-point quality commitment to consolidate the commitment to quality and purity and believe these minimum standards should apply to all recombinant protein manufacturers.
Qkine process controls:
- Full raw material traceability
- Complete SOP
- Dedicated protein purification column library (our manufacture columns are only ever used to purify the same protein)
- Industry-leading low endotoxin levels. Intriguingly we have seen recently high endotoxin in mammalian-expressed proteins due to storage of supernatants prior to purification, so it is critical endotoxin levels are reported irrespective of expression system.
Quality control performed on every lot:
- Single protein species and correctly folded bioactive protein evidenced by SDS-PAGE and mass spectrometry
- Quantitative bioassay
More information on the Qkine Nine-point Quality Commitment
References
[1] Svraka, L, Abdallah, HB, Johansen, C (2025) When recombinant proteins go wrong: The hidden pitfall of recombinant protein contamination. Cytokine 186: 156830. https://doi.org/10.1016/j.cyto.2024.156830
[2] Olsen OE, Skjærvik A, Størdal BF, Sundan A, Holien T (2017) TGF-β contamination of purified recombinant GDF15. PLoS ONE 12(11): e0187349. https://doi.org/10.1371/journal.pone.0187349
Qkine growth factors are manufactured to the highest quality standards and are tag free, carrier protein free and free from animal-derived contaminants, delivering low endotoxicity and high purity. All our proteins conform to our Nine-point Qkine Quality Commitment.
At Qkine, we are committed to raising the standards of growth factors, cytokines and related proteins to better support long-term and complex cell cultures. We are a science-led team, please reach out with any questions or requests to support@qkine.com.