Presentation by Luana Ferrara, Senior Protein Scientist
Video script
Slide 1: Introduction
Hello! My name is Luana Ferrara and I am a senior protein scientist at Qkine. In this short video, I’ll be presenting a comparative analysis of the purity of recombinant Activin A from different suppliers and highlighting why it’s important to consider the biochemical quality of growth factors for your stem cell culture.
Slide 2: The role of Activin A in stem cell culture
Recombinant Activin A protein is used in many human iPSC and ESC culture media for maintenance of pluripotency, as well as to induce stem cell differentiation primarily into endoderm lineages. As such a fundamental component of many stem cell media protocols, we were interested in benchmarking the purity of Activin A preparations against different suppliers.
Slide 3: Benchmarking of Activin A purity against different suppliers
SDS-PAGE is a useful tool for assessing the purity of growth factors; however, it is important that a sufficient amount of protein is loaded onto the gel and that a suitable stain is used to allow the detection of protein aggregates, degradation and unfolded products present at low concentrations within the preparation.
In this SDS-PAGE, you can see varying levels of protein aggregates, degradation and unfolded products present in the preparations from different suppliers. While these may not have a direct impact on your iPSC and ESC cultures, they can affect the concentration of Activin A in your cell culture media, leading to batch-to-batch inconsistency and less reproducible results.
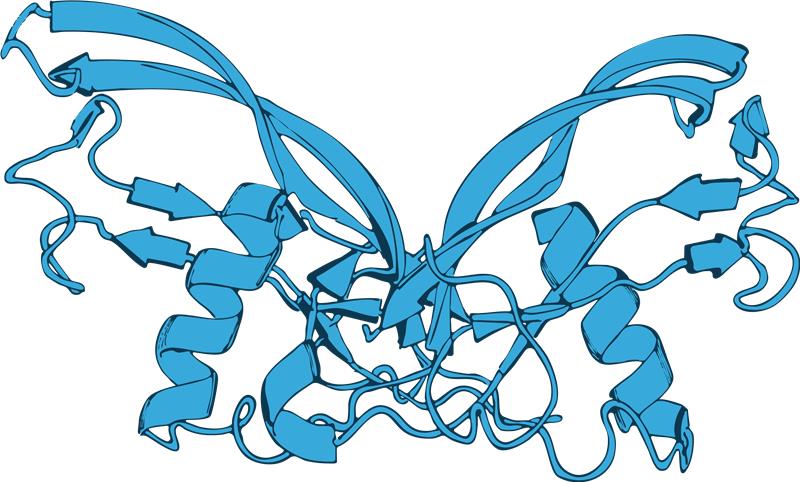
Slide 4: Confirmation of successful Activin A refolding after purification
As you can see from its structure, human Activin A protein is a 26 kDa disulfide-linked homodimer. In order to check that the protein has correctly refolded, SDS-PAGE needs to be performed under both reduced and non-reduced conditions. In the reduced SDS-PAGE, you can see a band at 13 kDa corresponding to the Activin A monomer, while in the non-reduced SDS-PAGE, a 26 kDa band confirms the protein has refolded correctly to form homodimers. To further confirm that the protein has refolded correctly and is functional, it’s also important to use a quantitative bioassay, such as an Activin A luciferase-responsive reporter assay.
Slide 5: Impact of growth factor purity and bioactivity on stem cell cultures
But why should you care about the purity and bioactivity of a growth factor like Activin A that is used at relatively low concentrations of your final media preparation?
If your growth factor purity and bioactivity isn’t as expected it can lead to:
- Less reproducible results from batch to batch, wasting valuable research time, money and effort.
- Potential off-target effects from low-level contaminants.
- Challenges when scaling culture conditions for applications such as tissue engineering.
Slide 6: About Qkine
That’s why at Qkine we’re on a mission to re-define industry standards for growth factor and cytokine biochemical quality by performing rigorous purity and bioactivity testing, for peace of mind that you’re using the highest quality, most reliable proteins for your stem cell cultures.
Slide 7: Thank you
Thank you for watching! If you have any questions or would like more information about our products, please get in touch or visit our catalogue page.