Maintenance of iNeurons using Qkine animal origin-free BDNF
In collaboration with Tong Li, Omer Bayraktar, and Maryna Panamarova
Wellcome Sanger Institute
Introduction
Diseases affecting the nervous system have been traditionally challenging to research, given the complexity of neural tissue and the limited translational applicability of model systems. These include age-related cognitive disorders, including Alzheimer’s and Parkinson’s diseases; neurological and psychiatric disorders, including depression and schizophrenia; and brain cancer [1]. Recent exciting advances in the in vitro use of human induced pluripotent stem cells (iPSCs) have provided valuable model systems to study neural development, disease mechanisms, and drug treatment.
iPSCs can be directed to differentiate into neural stem cells and subsequently into many neural and glial cell types by exposing them to specific small molecules, including growth factors and cytokines. Recent advances have demonstrated an alternative approach to directly convert iPSCs into functionally mature motor neurons and oligodendrocytes more efficiently and in a shorter time [2]. This process is referred to as inducible forward reprogramming and involves forcing the expression of transcription factors. At the Wellcome Sanger Institute, the transcription factor NGN2 was stably integrated under a doxycycline promoter using the Opti-OX technology [3]. The cortical neurons derived using this transcription-factor-based differentiation are termed iNeurons.
A component of the iNeuron media is Brain-Derived Neurotrophic Factor (BDNF), a member of the neurotrophin family that plays a crucial role in neural development, maintenance, and function [4,5]. BDNF stimulates neurogenesis and is also a significant regulator of synaptic plasticity and neuroprotection [6]. In the culture of iNeurons, BDNF supports their survival and growth, promotes axonal elongation and dendritic branching, and generates synaptic connections and structural neural networks [2]. Thus, it is essential to use high-quality and biologically active recombinant proteins to maintain the neural phenotype of the iNeurons. This application note reports on the use of high purity, animal origin-free BDNF (Qk050) for the maintenance of iNeurons.
References
[1] Zhao, X. & Moore, D. Neural stem cells: developmental mechanisms and disease modeling. Cell Tissue Res. 371, 1–6 (2018). doi: 10.1007/s00441-017-2738-1
[2] Fernandopulle, M. S. et al. Transcription-factor mediated differentiation of human iPSCs into neurons. Curr. Protoc. Cell Biol. 79, e51 (2018). doi: 10.1002/cpcb.51
[3] Pawlowski, M. et al. Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes. Stem Cell Rep. 8, 803–812 (2017). doi: 10.1016/j.stemcr.2017.02.016
[4] Maisonpierre, P. C. et al. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron 5, 501–509 (1990). doi: 10.1016/0896-6273(90)90089-x
[5] Skaper, S. D. The Neurotrophin Family of Neurotrophic Factors: An Overview. in Neurotrophic Factors: Methods and Protocols (ed. Skaper, S. D.) 1–12 (Humana Press, 2012). doi: 10.1007/978-1-61779-536-7_1
[6] Bramham, C. R. & Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 76, 99–125 (2005). doi: 10.1016/j.pneurobio.2005.06.003
Methods
Maintenance of iPSC line
KOLF2 iPSCs from the hipsci bank were transduced with an inducible NGN2 transgene at the Sanger Institute. The NGN2 KOLF2 iPSCs were cultured in E8 basal medium on vitronectin-coated 6-well plates and passaged using EDTA.
Generation and Culture of NGN2 iNeurons
On day 0, the NGN2 iPSCs were detached using accutase and resuspended as a single cell suspension in E8 with 10 µM rock inhibitor and 1 µg/ml doxycycline (Figure 1). The cells were seeded on a 6-well plate pre-coated with Geltrex at a density of 25,000 cells / cm2. On days 1 and 2, the media was replaced with fresh doxycycline-neural induction media A (dox-NIM A) – DMEM/F12 medium supplemented 1x N2 supplement, 1x GlutaMAX, 1x non-essential amino acids, 50 µM 2-Mercaptoethanol and 1 µg/ml doxycycline. On day 3, the iNeurons were passaged using accutase and resuspended in dox-NIM B (Neurobasal Plus Medium supplemented with 1x B27 plus, 1 µg/ml doxycycline, 10 ng/ml NT-3 ml and 10 ng/ml Qkine BDNF (Qk050)). The cells were seeded at a density of 10,000 cells / well of a 96-well plate pre-treated with 100 μg/ml PDL hydrobromide and coated with Geltrex. On days 4 to 6 of differentiation, 75% of the media volume was replaced daily with fresh dox-NIM B without disturbing the neurons. From day 8 of the differentiation, half the media volume was changed every other day with new dox-NIM B to limit the neuron exposure to air, as this affects cell quality and attachment.
Immunocytochemistry
On day 18 of differentiation, the iNeurons were fixed with 4% formaldehyde, washed with PBS and blocked with 10% donkey serum for 2 h at room temperature (RT). The cells were incubated with the primary antibodies Rat Anti-Tublin (YOL1/34, ab6161, abcam) diluted in 1% donkey serum for 2 h at RT then secondary and primary conjugate antibodies PSD-95 Antibody (6G6-1C9, Janelia Fluor 549, Novus) and Alex Fluor 647 Anti-Synaptophysin (ab196166, abcam) with DAPI diluted in 1% donkey serum for 2 h at RT. Images were acquired using the Operetta CLS (Perkin Elmer) and viewed using the OMERO Plus image database (Glencoe Software).

Figure 1. Schematic diagram of the methodology for iNeuron differentiation and imaging assay (Diagram created on Biorender). On day 0, NGN2 iPSCs are seeded on Geltrex and cultured in dox-NIM A until day 3. On day 3, the iNeurons are passaged and resuspended in dox-NIM B medium in a 96 well plate. The iNeurons are fixed and stained on day 18 of differentiation and imaged using the Operetta CLS.
Results
iNeurons formed structural networks and expressed synaptic markers
By day 18, the iNeurons developed long neurites, fully matured, and established synaptic connections, forming structural neural networks (Figures 2 and 3). The iNeurons were further characterized and stained for the nucleus tubulin synaptophysin, and postsynaptic density protein-95 (PSD-95). The neurites of the iNeurons expressed tubulin, confirming the presence of axons. The iNeurons were also positive for presynaptic (synaptophysin) and postsynaptic (PSD-95) markers. Synaptophysin and PSD-95 both showed a stippled pattern. Synaptophysin was expressed in the cell body of the iNeurons (Figure 2) whereas PSD-95 was mainly expressed on the neurites (Figure 3).
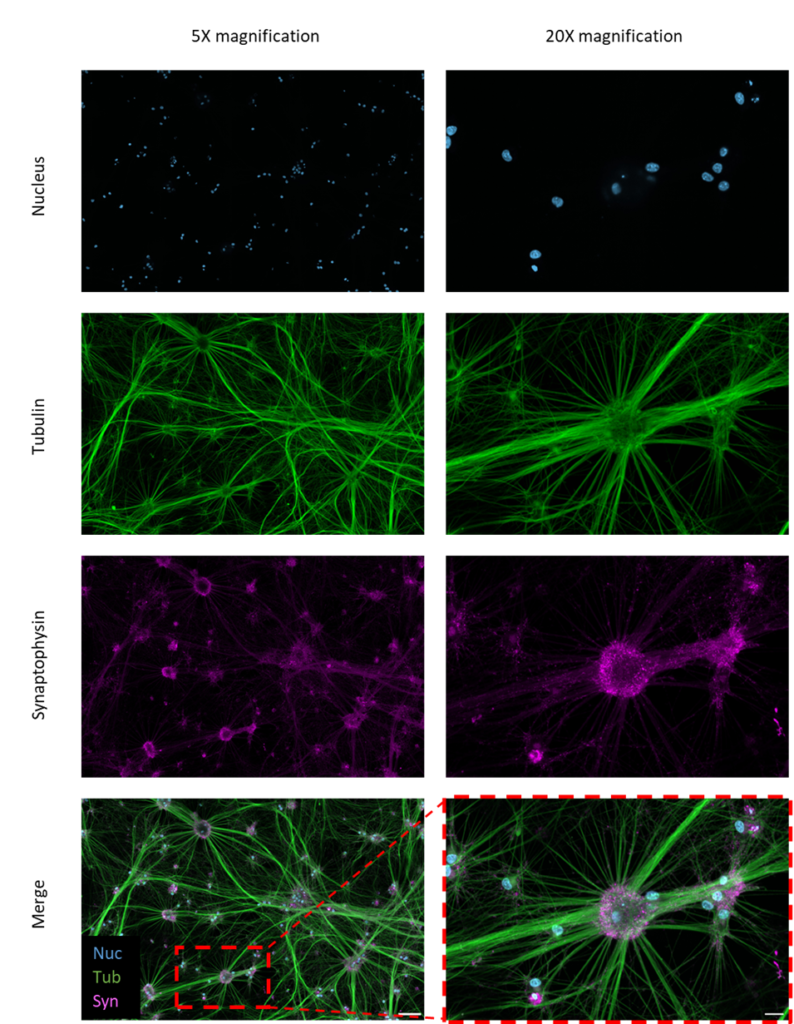
Figure 2 – Immunofluorescence images of iNeurons stained for presynaptic neural markers. iNeurons were stained for the nucleus (blue), tubulin (green), and synaptophysin (purple). Scale bars = 100 µm (5x magnification) and 20 µm (original imaging at 20x magnification).
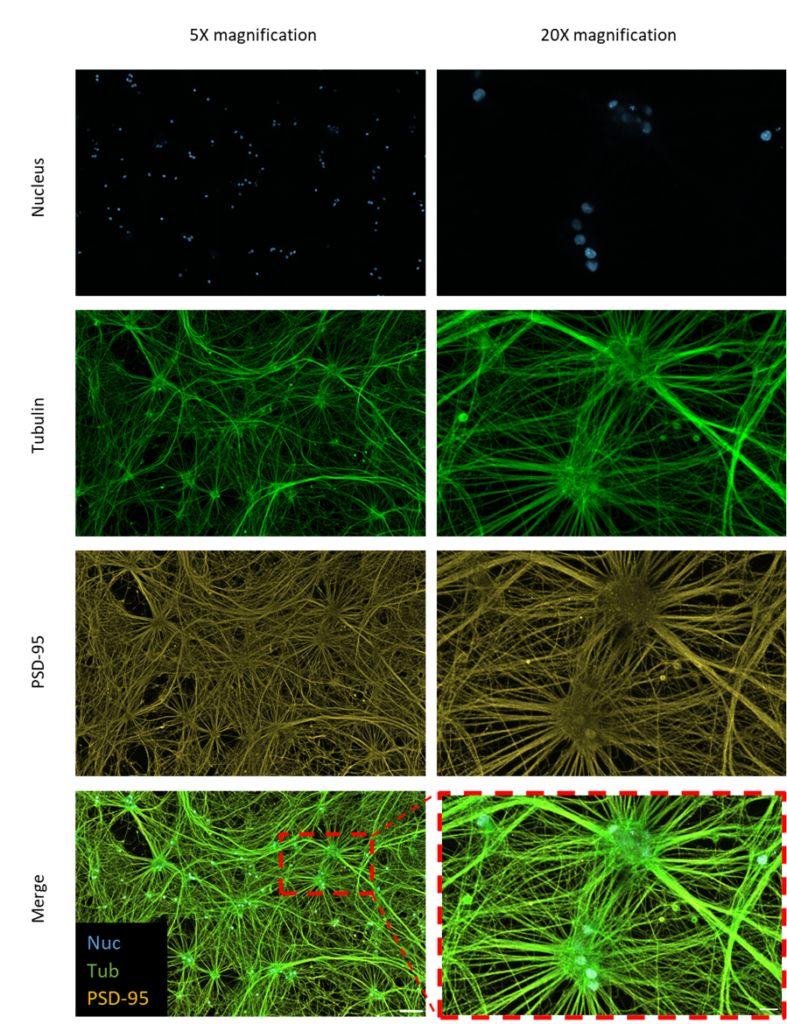
Figure 3 – Immunofluorescence images of iNeurons stained for postsynaptic neural markers. iNeurons were stained for the nucleus (blue), tubulin (green), and PSD-95 (yellow). Scale bars = 100 µm (5x magnification) and 20 µm (original imaging at 20x magnification).
Conclusion
iNeurons cultured with Qkine animal-free BDNF (Qk050) developed long neurites and formed synaptic connections. They maintained a neuronal phenotype with the expression of neuron-specific, presynaptic, and postsynaptic markers.
Using animal origin-free proteins to culture neural stem cells, including iNeurons, reduces variability observed in animal-derived materials. This ensures a more controlled environment and reproducible differentiation. With the increasing demand for iNeuron-like cells, the ability to produce neurons on a larger scale becomes essential. Animal origin-free proteins meet the scale-up challenges of culturing iNeurons as they can be produced in bulk, whilst remaining highly pure and bioactive. Integrating animal origin-free recombinant proteins into the culture of iNeurons ensures that these cultures can be translated to downstream clinical applications if required.
Further Information
Qkine growth factors are manufactured to the highest of quality standards and are free from animal-derived contaminants, delivering low endotoxicity and high purity. At Qkine, we are committed to raising the standards of growth factors, cytokines and related proteins to better support long-term and complex neural stem cell culture. We are a science-led team, please reach out with any questions or requests to support@qkine.com.
All our recombinant proteins are animal-free and come with Bioactivity. Guaranteed.
Featured resources
Contact us
Our science team is here to help, please contact us if you have any questions.