Quality commitment 1
Certified animal origin-free proteins with full traceability
We never compromise on the purity and bioactivity of our proteins. To ensure
full reproducibility and enhance scientific outcomes, all our products adhere to the
Nine-point Qkine Quality Commitment.
High-quality, animal origin-free protein manufacturing
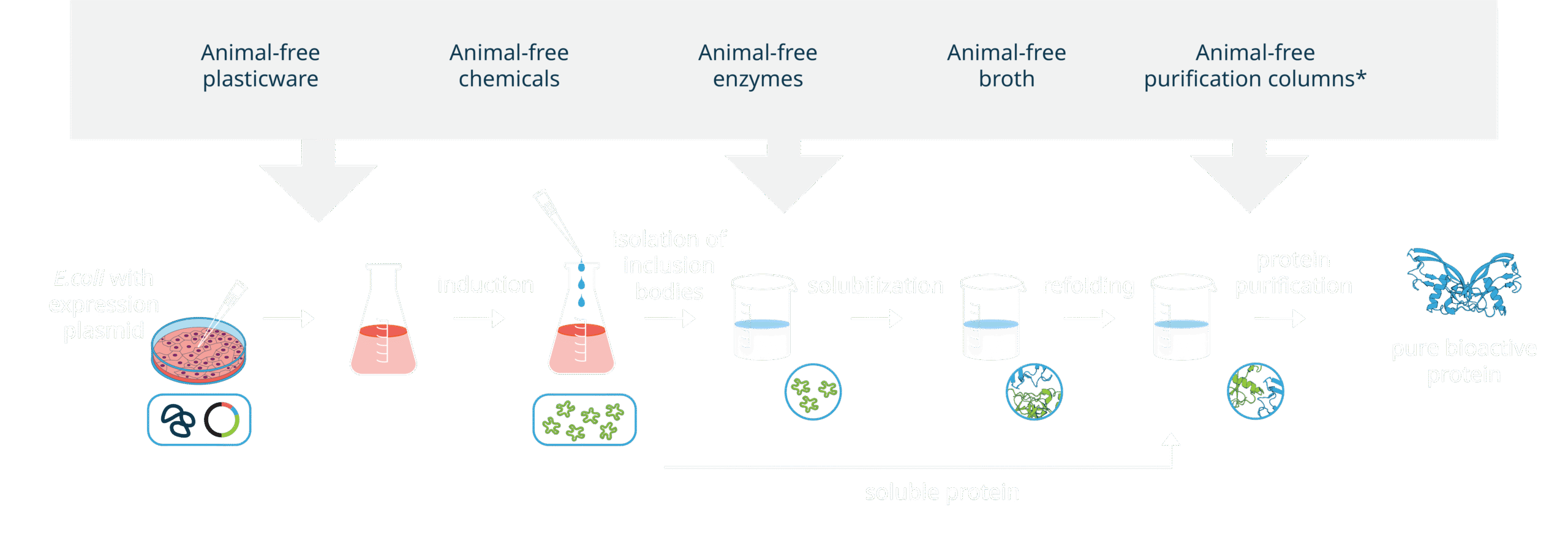
All our products are AOF (animal-origin free). Each production lot undergoes rigorous testing across various quality control assays to ensure a consistently high level of quality for every lot. This includes the industry’s lowest endotoxin level pass criteria, as well as mycoplasma and sterility testing, as standard. Additionally, our products are free from BSE/TSE.
Proteins are manufactured in an E. coli microbial expression system, with no use of baculovirus on-site.

Why use Qkine animal origin-free growth factors and cytokines for your stem cell cultures?
Consistency & reliability enhanced
Utilizing animal origin-free recombinant proteins eliminates variations arising from trace animal components, promoting improved cell culture consistency. All our recombinant proteins mitigate the risk of contamination by mammalian pathogens, enhancing the reliability of cell products.
Excellent lot-to-lot consistency
All Qkine growth factors and cytokines have exceptional lot-to-lot consistency. Every lot is checked during a stringent QC process to ensure it has the same bioactivity as prior lots and to ensure reproducibility.
High level of purity with the lowest endotoxin level
We guarantee >98% purity due to assay limits, but we are confident that the true result is higher. To ensure purity, we always run an overloaded gel with 7 µg of protein to ensure that we can not visually detect any contaminants, aggregates, or degradants.
High biological activity
At Qkine, we test and validate the biological activity of all our growth factors against other suppliers’ proteins to ensure that we provide you with proteins of the highest level of biological activity.
Cost-effectiveness & scalability
Animal origin-free manufacturing offers greater supply chain stability and contributes to higher lot-to-lot consistency and reproducibility. This ensures that researchers can scale up production relying on consistent quality, reducing the need for extensive quality control measures and optimizing resource utilization.
Discover animal-free recombinant proteins today to improve reproducibility in your cultures
Animal origin-free manufacturing process control
As part of our ongoing commitment to quality in every aspect of the company, animal origin-free production is taken into consideration from the very earliest planning stages of design and development. Before being incorporated into a procedure, newly-sourced materials must be reviewed and approved by our Quality team.
We continuously work with our suppliers to confirm and certify that the chemical components used within the manufacture of Qkine products are derived from non-animal and non-human sources and that the processes and components used in their own manufacture are animal-free.
Every stage of the Qkine animal-free protein manufacturing employs Standard Operating Procedures (SOPs). SOPs are fundamental to assure consistency from lot to lot and guarantee the highest quality of products.
Qkine is ISO 9001:2015 certified for development, manufacture, and supply of proteins and related products.
Every component used during the manufacture of our products is assessed by our Quality team.

Animal-free certificates, certificates of origin (manufacture details), certificates of analysis (quality and bioactivity information) and MSDS are available for all products. Please request any documentation below.
Quick guide: AOF or ADCF?
| Difference between animal-origin free (AOF) & animal-derived component free (ADCF) | AOF | ADCF |
|---|---|---|
| Raw materials used directly in the product or as a starting material (e.g. E.coli growth media) are not derived from animal or human tissue, cells or body fluids | ✔ | ✔ |
| Raw materials used directly in the product or as a starting material do not contain animal or human tissue, cells, or body fluids at any stage of their manufacture process | ✔ | ✔ |
| Consumables used within the production process (e.g. filters) are not derived from animal or human tissue, cells or body fluids and no animal or human tissue, cells or body fluids are used at any stage of their manufacture process. | ✔ | ✗ |
Contact us
Our dedicated team of stem cell specialists is available to help and answer any questions you have about specific products, processes and our animal-free ethos.