Quality commitment 7
Assayed to ensure industry-leading low endotoxin levels
At Qkine we impose an industry-leading internal standard for endotoxin levels of <0.1 EU per µg protein for all our proteins.
Minimising the introduction of endotoxins
At Qkine our in-house R&D team work to the highest standards to minimise endotoxin contamination.
High quality management processes.
Good aseptic technique and filter sterilization of buffers used during manufacturing.
Rigorous purification column cleaning protocols.
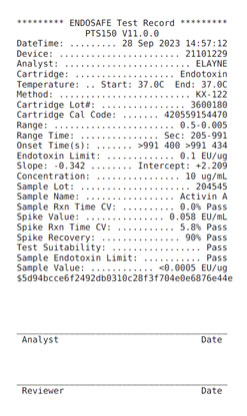
Each lot tested using the Limulus Amebocyte Lysate (LAL) assay.
Endotoxins can affect the growth or performance of cell cultures and are a major source of experimental variation.
To determine the presence of endotoxins, a Limulus Amebocyte Lysate (LAL) assay is used. This assay can detect as little as 0.01 endotoxin units (EU)/ml. One EU equals approximately 0.1 to 0.2 ng endotoxin/ml of solution.
Current industry standards require endotoxin levels in growth factor lots to be less than 0.5 EU/ml, however the lower the better, and companies such as Qkine impose internal standards of <0.1 EU per µg protein.
The majority of Qkine growth factors exhibit endotoxin levels below the limit of detection (<0.0005 EU/μg protein in standardised FDA-compliant assays).
Qkine have also benchmarked mammalian-expressed proteins from alternative supplier, which should theoretically contain no endotoxin, and measured endotoxin levels exceeding the threshold of 0.25 EU/μg.
Getting the simple things right is important.
At Qkine, we are committed to raising the standard in bioactive protein manufacturing.
Our science team is here to help, please contact us if you have any questions at customerservice@qkine.com.
Endotoxins
Endotoxins are small hydrophobic lipopolysaccharide molecules, toxic substances found in the outer cell membrane of gram-negative bacteria. Endotoxins are shed by bacteria during their cell death or when they are actively growing and dividing. Endotoxins have a very high heat stability meaning they cannot be destroyed with regular decontamination methods such as autoclaving. Endotoxins are also hydrophobic, and consequently have a strong affinity for other hydrophobic materials such as lab plastics. Endotoxin introduction can be avoided by cleanliness and proper lab techniques to keep endotoxin levels in a lab at bay.
At Qkine, we achieve this with good aseptic technique, filter sterilization of buffers used during manufacturing processes, and rigorous purification column cleaning protocols. This minimizes the introduction of endotoxins into any manufacturing/research processes.
Limulus Amebocyte Lysate (LAL) assay
The Limulus Amebocyte Lysate (LAL) assay uses an extract of blood cells (amoebocytes) obtained from the horseshoe crab (Limulus polyphemus). These blood cells were found to clot in the presence of endotoxin, and this technology was used to develop endotoxin detection assays.
LAL assay reagent reacts with the bacterial endotoxins or lipopolysaccharide (LPS).
Raising the standard in bioactive protein manufacturing and innovation
Our science team is here to help, please contact us if you have any questions.