Hematopoietic stem cells (HSCs) are at the forefront of a transformative shift in modern medicine, beginning to revolutionize patient care by offering novel therapeutic treatments for a wide range of diseases. HSCs are advancing treatments for hematological conditions such as leukemia and lymphoma and offering hope for regenerative therapies in conditions such as autoimmune diseases and genetic disorders. The versatility and potential for personalized treatment strategies represent a significant leap forward that could reshape how we approach complex health challenges.
Hematopoietic Stem Cells
Hematopoietic stem cells are multipotent progenitors primarily located in the bone marrow. They possess the ability to differentiate into all blood cell lineages, including erythrocytes, leukocytes, and thrombocytes. HSCs are essential for the continuous regeneration of the hematopoietic system, ensuring the maintenance of hemostasis and immune function. Cutting-edge advancements, including CRISPR-Cas9-mediated gene editing, cell isolation techniques, and insights into the HSC niche microenvironment, have exponentially increased the therapeutic viability of HSCs. HSCs have a critical role in the reconstitution of the hematopoietic system after hematopoietic stem cell transplantation.

Hematopoietic Stem Cell Transplantation
Hematopoietic Stem Cell Transplantation (HSCT) has emerged as a key therapy for hematological malignancies such as leukemia and lymphoma, and bone marrow failure syndromes like aplastic anemia. HSCT functions by replacing the defective hematopoietic system with healthy HSCs, enabling sustained remission and improving patient prognosis.
There are two primary types of HSCT, autologous and allogeneic transplantation. In autologous HSCT, patients receive their own HSCs, which have been harvested and stored prior to undergoing intensive treatment, such as chemotherapy or radiation. This approach is often used to rescue the patient’s hematopoietic system after these treatments, reducing the risk of relapse and minimizing complications. In allogeneic HSCT, commonly referred to as bone marrow transplant, patients receive HSCs from a donor—either related or unrelated—whose tissue type closely matches their own. This form of transplantation is particularly effective in treating hematological malignancies, as the donor’s immune cells can also help target and eliminate residual cancer cells, a phenomenon known as the graft-versus-tumor effect.
One of the critical challenges in HSCT has been the limited availability of suitable HSCs, particularly for allogeneic transplantation where finding a compatible donor can be difficult. Recent advancements in the expansion of HSCs have been instrumental in overcoming this barrier. The development of small molecule compounds such as polyvinyl alcohol (PVA) substrates and innovative culture techniques has significantly enhanced the ability to expand HSCs ex vivo, making it possible to generate a larger number of viable cells for transplantation. This expansion technology not only improves the availability of HSCs but also enhances engraftment success rates and reduces the risk of complications such as graft failure or delayed recovery.
Enhancing the expansion capacity of HSCs for HSCT
One of the key areas of development in HSCT research is the use of polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (PCL-PVAc-PEG) to enhance the proliferation of HSCs for HSCT. This polymer has shown superior cell proliferation potential compared to traditional PVA substrates. When combined with specific compounds like 740Y-P, butyzamide, and UM171, this medium significantly increases the expansion capacity of human HSCs by 75-fold and CD34+ cells expanded 55-fold.
Additionally, there is significant current research utilizing ex vivo-expanded cord blood (CB) products such as NiCord. For example, in one Phase I trial, patients receiving NiCord, expanded CD133+ cells cultured with nicotinamide (NAM), demonstrated significantly faster neutrophil engraftment compared to historical controls. A subsequent Phase III trial confirmed these findings, showing that omidubicel (formerly NiCord) significantly reduced neutrophil recovery time and infection rates post-transplantation. The FDA has recently approved omidubicel for patients with hematologic malignancies, marking a milestone in the clinical application of ex vivo-expanded HSCs.
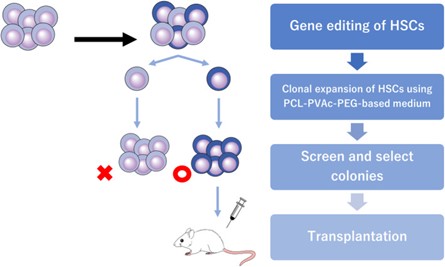
Figure 1: Scheme for controlling the genetic heterogeneity in gene‐edited murine hematopoietic stem cells (HSC) by single‐cell culture. PCL‐PVAc‐PEG, polyvinyl caprolactam‐acetate polyethylene glycol.
Credit: Sakurai, M., et al. Ex vivo expansion of human hematopoietic stem cells and clinical applications. Cancer Sci. 115, 698–705 (2024).
These advancements underline the transformative potential of ex vivo HSC expansion technologies. By optimizing culture conditions and employing innovative materials and compounds, researchers are overcoming previous limitations in HSC expansion. This progress not only enhances the availability of HSCs for transplantation but also improves patient outcomes, indicating a promising future for HSC-based therapies in treating various hematologic conditions.
Despite these advancements, HSCT is not without its risks. Complications such as graft-versus-host disease (GVHD), where the donor immune cells attack the recipient’s tissues, remain a significant challenge. Ongoing research is focused on developing strategies to prevent and treat GVHD, including the use of regulatory T cells and other immunomodulatory approaches.
HSCs represent a remarkable advancement in medical science, with the potential to reshape the landscape of therapeutic treatments for a wide range of diseases from their critical role in hematopoietic stem cell transplantation to their emerging applications in gene therapy and immunotherapy. As research continues to unlock the full potential of HSCs, their transformative impact on medicine is set to grow, paving the way for more personalized, effective, and curative treatments in the future.
Related reading
Cancer Sci. 202
Ex vivo expansion of human hematopoietic stem cells and clinical applications.
Authors: Sakurai, M., Ishitsuka, K., Becker, H. J. & Yamazaki, S.
BBMT 2017.
Transplantation of Ex Vivo Expanded Umbilical Cord Blood (NiCord) Decreases Early Infection and Hospitalization.
Authors: Anand S., Thomas S., Hyslop T., Adcock J. et al.
Annual review of immunology 2021.
Current concepts and advances in graft-versus-host disease immunology.
Authors: Hill, Geoffrey R. et al.
J Exp Med. 2021.
Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection.
Authors: Caiado, F., Pietras, E. M. and Manz, M. G.