Recombinant human/mouse/rat Activin A PLUS ™ protein (Qk005)
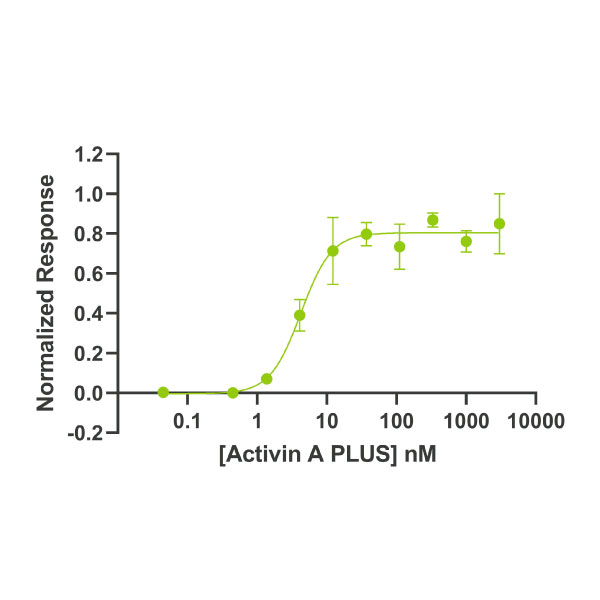
Human/mouse/rat Activin A PLUS ™ protein is an optimised biologically active truncation of the mature domain of human Activin A protein. The EC50 and activity in stem cell culture of activin A PLUS is equivalent to the full mature domain activin A.
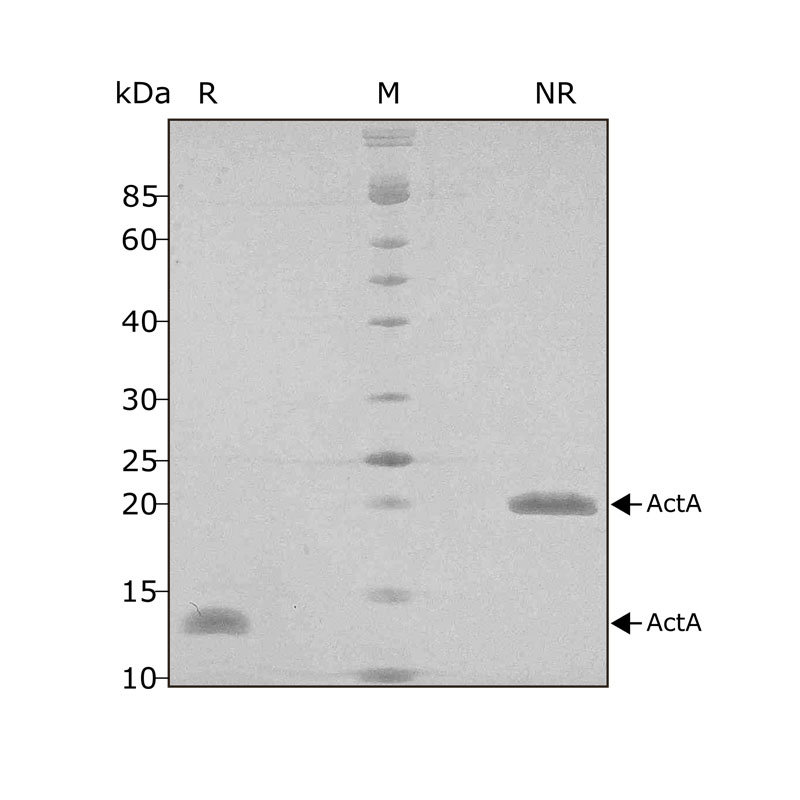
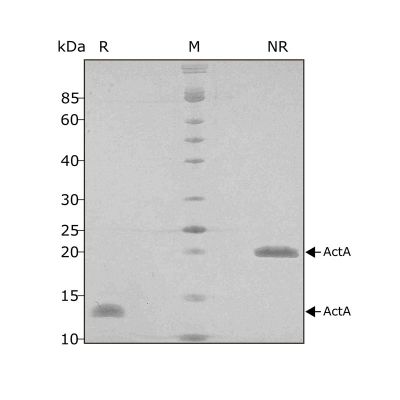
High purity 24 kDa dimer comprising truncated mature domain of activin A protein, animal-free (AF) and carrier-protein free (CF). Recombinant Activin A PLUS is designed to be manufactured at scale for cost-effective, large-scale stem cell culture applications.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.


Potent and stable Activin A PLUS & FGF2-G3
tianmingwu –
We were seeking for a good quality replacement for mammalian cell host human growth factors and glad that we chose Qkine. The Activin A PLUS and FGF2-G3 performed better for H9-hESC self-renewal and primitive germ layer differentiation.
Upvote if this was helpful (2) Downvote if this was not helpful (0) Flag for removal