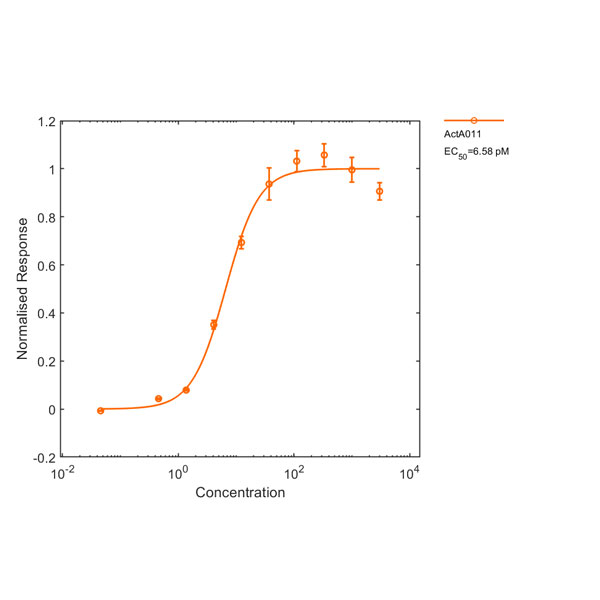
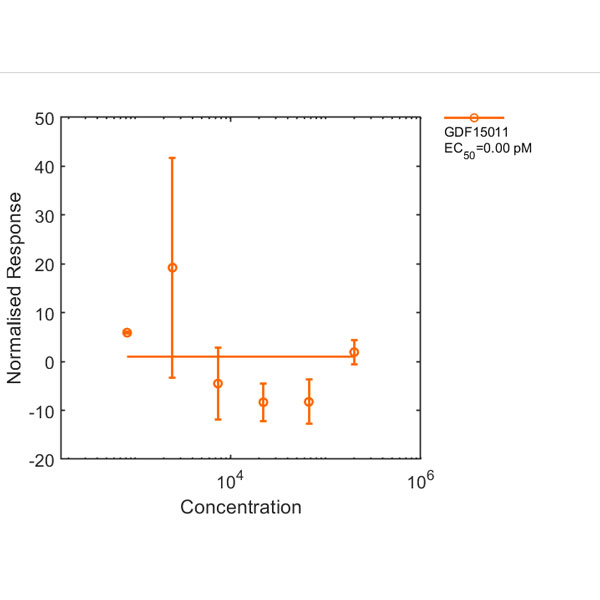
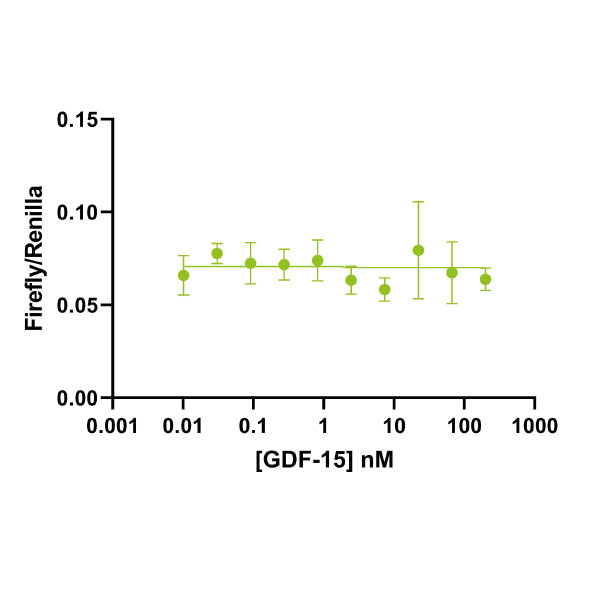
Recombinant human GDF-15 protein (Qk017)
Human GDF15 (growth differentiation factor 15) protein is a member of the TGFβ family and subject of intense interest as a marker of cellular stress and for its role in metabolism, cancer and pregnancy. Human GDF15 also is functional in mouse studies.
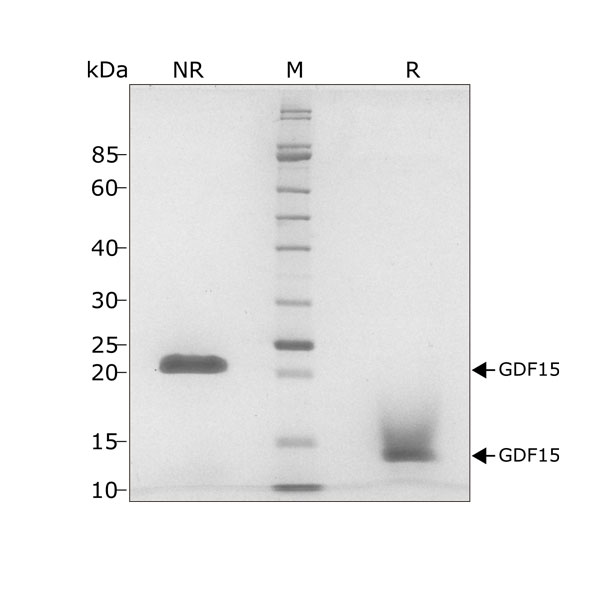
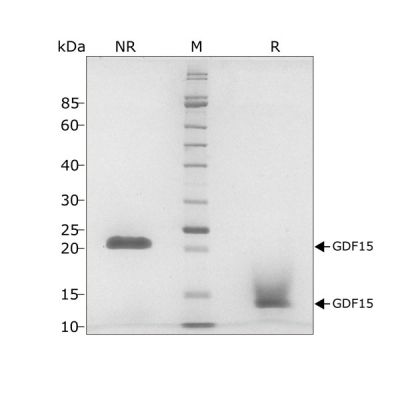
This 25 kDa disulfide-linked dimer is composed of the mature domain of human growth differentiation factor 15 protein. Our recombinant GDF15 protein is expressed in E. coli and exceptionally high purity extensively validated to ensure no trace contamination of related TGFβ family proteins from the mammalian culture systems, which historically led to controversy in the literature.
Our products are for research use only and not for diagnostic or therapeutic use. Products are not for resale.



What others are saying
There are no contributions yet.